Team:Amsterdam/extra/protocols
From 2012.igem.org




Protocols
Transformation Protocol in DH5a (Invitrogen)
- Thaw an aliquot of competent bacteria on ice.
- Add 50 µl of DH5a competent cells gently in a sterile 15 ml polypropylene tube.
- Add 1 µl of ligation mixture or Gibson Assembly reaction (1 – 10 ng of DNA) to the polypropylene tube
- Incubate on ice for 30 minutes.
- Heat shock for 45 seconds in a water bath at 42°C, then quickly back on ice for 2 minutes.
- Add 450 ml of room temperature SOC medium (Work sterile!!).
- Incubate 1 hour at 37°C for antibiotic resistance expression, while shaking (225 rpm).
- Spread 1/10 and 9/10 on LB plates (containing the right antibiotic).
Notes:
- Do not shake the polypropylene tubes during the heat shock!
- To increase the yield, centrifuge gently (at low speed), remove the excess of medium and then spread on the plates.
- To determine transformation efficiency
Gel Electrophoresis
- Dissolve 2g of Agarose in 200 ml TAE or TBE buffer. Heat until the solution is clear. Do not boil.
- Allow to cool down and add 2µl of ethidium bromide.
- Transfer to the casts + combs and leave at room temperature. Store at 4°C for later use.
- Input:
- 5 µl DNA ladder
- 5 µl sample + 5 µl H2O + 1 µl loading buffer
Preparation of LB Medium and Agar Plates
- Luria-Bertani (LB) medium is a nutritionally rich medium that can be used for the preparation of plasmid DNA and recombinant proteins. It is one of the most common media used for maintaining and cultivating recombinant strains of Escherichia coli.
- To 950 ml of deionised water, add:
- Bacto Tryptone – 10 grams
- NaCl – 10 grams
- Yeast extract – 5 grams
- Shake until all the solutes have completely dissolved. Adjust the pH to 7.0 with 5N NaOH (˜ 0.2 ml). Adjust the total volume to one litre with deionised water. For LB plates, add 15g/litre of Bacto agar to the medium before autoclaving.
Sterilise by autoclaving (standard autoclaving liquid cycle protocol).
- Allow to cool down and add the right amount of antibiotic.
- Add about 15 – 20 ml of solution to plates in a sterile environment. Allow to settle and store at 4°C.
Gibson Assembly
Preparation of reagents
- Preparation of 6ml of 5X isothermal reaction buffer by combining (This buffer can be aliquoted and stored at –20°C):
- 3 ml of 1M Tris-HCl pH 7.5
- 150 ml of 2M MgCl2
- 60 ml of 100mM dNTP
- 300 ml of 1M DTT
- 1,5 g PEG-8000
- 300 ml of 100mM NAD
One-step isothermal DNA assembly protocol (Gibson Reaction)
- For one reaction containing 40 µl:
- 8 µl 5X isothermal buffer
- 0.8 µl of 0.2 U.µl –1 or 1.0 U.µl –1 T5 exonuclease
- 4 µl of 40 U.µl –1 Taq DNA ligase
- 0.5 µl of 2 U.µl –1 Phusion DNA polymerase.
- 5 µl of DNA
- Water up to 40 µl
- 50 ºC ----- 1 hour
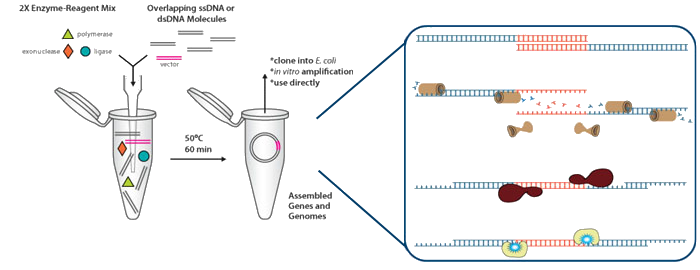
Adapted from:
http://www.syntheticgenomics.com
http://eu.idtdna.com
Notes:
- All isothermal assembly components can be stored at –20°C in a single mixture at 1.33X concentration for more than one year. The enzymes are still active after more than ten freeze-thaw cycles. The aliquots should be kept on ice until ready to use.
- The exonuclease amount is ideal for the assembly of DNA molecules with 20– 150 bp overlaps.
- Between 10 and 100 ng of each ?6 kb DNA fragment was added.
- For larger DNA segments, proportional amounts of DNA should be added (for example, 250 ng of each 150 kb DNA segment).
DNA Precipitation
- Add 1/10 of sodium acetate (3M, pH 5.2) to the total volume of DNA to be precipitated. Vortex to make sure that the solution is well mixed.
- Add 2.5 X total volume in eppendorf of 100% ethanol. Vortex and keep the mixture at -20°C for 30 minutes.
- Centrifuge at maximum speed for 20 minutes.
- Discard the supernatant without disturbing the pellet and add about 500 µl of 70% ethanol.
- Centrifuge for one minute at maximum speed. Discard the supernatant.
- Add about 500 µl of 70% ethanol again. Centrifuge for one minute at maximum speed. Discard the supernatant.
- Remove as much ethanol as possible using a glass Pasteur pipette. Allow to air dry.
- Resuspend the DNA in 20 µl of deionised sterile water.
Notes:
- Keep the 100% ethanol at -20°C. The ethanol must always be cold prior to use.
- Centrifugation at 4°C is preferable.
Preparation of Competent E. coli
Preparation of Reagents
- Buffer 1:
- 30 mM KOAc, 100 mM RbC12, 10 mM CaCl2, 50 mM MnC12, 15% glycerol, pH 5.8
- For 500 ml: 1.47 g KOAc (MW 98.14)
- 6.04 g RbC12 (MW 120.92)
- 0.74 g CaC12 (MW 147.02)
- 4.94 g MnC12-4H20 (MW 197.9)
- 75 ml glycerol
- pH to 5.8 with dilute acetic acid.
- Filter sterilize
- Buffer 2:
- 10 mM MOPS/KOH pH 6.5, 75 mM CaC12, 10 mM RbC12, 15% glycerol
- For 100 ml: 0.21 g MOPS (MW 209.26)
- 1.10 g CaC12 (MW 147.02)
- 0.12 g RbC12 (MW 120.92)
- 15 ml glycerol
- pH to 6.5 with 1 N KOH.
- Filter sterilize.
Protocol
- Streak desired E. coli strain on fresh LB plate. Grow overnight at 37°C.
- Inoculate a single colony into 5 ml LB. Grow overnight, shaking at 37° C.
- Inoculate about 1 ml into 200 ml LB in a 2 1 flask. Shake at 37°C until OD550 = 0.5.
- Chill flask in ice-water 5 minutes.
- Spin 5 minutes at 6,000 rpm in GS3 rotor.
- Resuspend pellet in 80 ml ice cold Buffer 1.
- Chill in ice-water 5 minutes.
- Spin 5 minutes at 6,000 rpm in GS3 rotor.
- Resuspend pellet in 8 ml ice cold Buffer 2.
- Chill in ice-water 15 minutes.
- Make 50, 100 and 200 ml aliquots in 1.5 ml Eppendorf tubes.
- Flash-freeze in liquid nitrogen.
- Store at -80 degrees.
Notes:
- Keep buffers, tips, tubes, rotors, etc. ice cold
Long time exposure protocol
Day 1
- 1. End of the day: Prepare and incubate (37C) 6x 3ml pSB1AT3+LacH containing bacterial cultures (with appropriate antibiotic)
- 2. End of the day: Prepare and incubate (37C) 6x 3ml pSB1AT3+pBAD containing bacterial cultures (with appropriate antibiotic)
Day 2
- 1. Start of the day: To 3 cultures from Day 1, Step 1 add to each 15 ul of 1M IPTG solution
- 2. Start of the day: To 3 cultures from Day 1, Step 2 add to each 120 ul of 100% (1g/ml) Arabinose solution
- 3. Keep all cultures incubated at 37C
Day 3
- 1. Start of day: From 1 of the cultures from Day 2, Step 1 take 2x a 1.65ml sample (1 for miniprep 1 for western blot), discard rest of culture (IPTG+ 1 day exposure)
- 2. Start of day: From 1 of the cultures from Day 2, Step 2 take 2x a 1.65ml sample (1 for miniprep 1 for western blot), discard rest of culture (IPTG- 1 day negative control)
- 3. Start of day: From 1 of the cultures from Day 1, Step 1 (and not used in Day 2, Step 1) take 2x a 1.65ml sample (1 for miniprep 1 for western blot), discard rest of culture (ARA+ 1 day exposure)
- 4. Start of day: From 1 of the cultures from Day 1, Step 2 (and not used in Day 2, Step 2) take 2x a 1.65ml sample (1 for miniprep 1 for western blot), discard rest of culture (ARA- 1 day negative control)
- 5. Start of day: To the 2 remaining cultures of Day 2, Step 2 each add 120 ul of 100% (1g/ml) Arabinose solution
- 6. Keep all (remaining) cultures incubated at 37C
Day 4
- 1. Start of day: From 1 of the (remaining) cultures from Day 2, Step 1 take 2x a 1.65ml sample (1 for miniprep 1 for western blot), discard rest of culture (IPTG+ 2days exposure)
- 2. Start of day: From 1 of the (remaining) cultures from Day 2, Step 2 take 2x a 1.65ml sample (1 for miniprep 1 for western blot), discard rest of culture (IPTG- 2 days negative control)
- 3. Start of day: From 1 of the (remaining) cultures from Day 1, Step 1 (and not used in Day 2, Step 1) take 2x a 1.65ml sample (1 for miniprep 1 for western blot), discard rest of culture (ARA+ 2 days exposure)
- 4. Start of day: From 1 of the (remaining) cultures from Day 1, Step 2 (and not used in Day 2, Step 2) take 2x a 1.65ml sample (1 for miniprep 1 for western blot), discard rest of culture (ARA- 2 days negative control)
- 5. Start of day: To the 1 remaining culture of Day 2, Step 2 add 120 ul of 100% (1g/ml) Arabinose solution
- 6. Keep all (remaining) cultures incubated at 37C
Day 5
- 1. Start of day: From the 1 remaining culture from Day 2, Step 1 take 2x a 1.65ml sample (1 for miniprep 1 for western blot), discard rest of culture (IPTG+ 3 days exposure)
- 2. Start of day: From the 1 remaining culture from Day 2, Step 2 take 2x a 1.65ml sample (1 for miniprep 1 for western blot), discard rest of culture (IPTG- 3 days negative control)
- 3. Start of day: From the 1 remaining culture from Day 1, Step 1 (and not used in Day 2, Step 1) take 2x a 1.65ml sample (1 for miniprep 1 for western blot), discard rest of culture (ARA+ 3 days exposure)
- 4. Start of day: From the 1 remaining culture from Day 1, Step 2 (and not used in Day 2, Step 2) take 2x a 1.65ml sample (1 for miniprep 1 for western blot), discard rest of culture (ARA- 3 days negative control)
Exposure Window Experiment
Day 1
- Set in 2 o/n cultures of 10 ml with the psb1at3-lac/bad-mtase (mutated) with antibiotics
Day 2
- Set each o/n culture through in 4 different tubes. Take 2 ml and fill up to 3 ml with LB and antibiotics.
Day 3
- Give 15 ul of 1M IPTG and 1200 ul of 10% Arabinose creating;
- LacH; 1;normal (-), 2;normal(-), 3;signal(+), 4;signal(+)
- pBAD;1;normal (-), 2;normal(-), 3;signal(+), 4;signal(+)
Day 4
- Take 1 ml sample checking day 3 created settings
- Give again same IPTG and Arabinose creating;
- LacH; 1;normal (--), 2;signal(-+), 3;normal(+-), 4;signal(++)
- pBAD;1; normal (--), 2;signal(-+), 3;normal(+-), 4;signal(++)
Day 5
- Take 1 ml sample checking day 4 created settings
- Mini-prep all taken samples and digest with ScaI
Growth curve experiment 5 september
4 samples
- LacH promoter + Mtase in psb1at3 [with and without IPTG]
- pBAD promoter + Mtase in psb1at3 [with and without Arabinose]
The starting samples
- 100 (95*) ml LB broth
- 5 ml of O/N culture (one of two constructs creating a 5% state)
- antibiotic: Ampicilin ( 1% of total will make for 1 ml / Erlenmeyer )
One of each construct will be kept as Blanco and one of each construct will receive IPTG or Arabinose
IPTG (10mM) v Arabinose (~1% is generally used) [Meaning you will need 1 ml of a 10% stock]
Growth curve time points
The assessment Jose suggested seems fine.
This contained a time laps of 30min for the first period of time (lets say first 3 hours) and after that time lapses of a hour would be better.
Growth curve samples
- 1 ml is taken to measure OD
- 1 ml is taken for WB analysis
- 1 ml is taken for digest analysis
Degradation of Signal at varying exposure times and time points
Day 1 (13-9-2012)
Setup:
- Prepare 2x 10ml pSB1AT3 + LacH cultures (in normal LB and with appropriate antibiotic added)
- Prepare 2x 10ml pSB1AT3 + pBAD cultures (in normal LB and with appropriate antibiotic added)
- Incubate at approximately 37 C for 20 hours (starting time 21:20)
Day 2 (14-9-2012)
Setup:
- Stop incubation of cultures after 20 hours of growth (at 16:20), cultures should now be well into the stationary faze.
- Prepare 8x a 500ml flasks containing 49.5ml of LB each
- For 4 flasks add appropriate antibiotic for culture 1 (pSB1AT3 + LacH containing bacteria from day 1 - step 1)
- For 4 flasks add appropriate antibiotic for culture 2 (pSB1AT3 + pBAD containing bacteria from day 1 - step 2)
- To the 4 flasks from step 3 add 0.5ml of culture 1
- To the 4 flasks from step 4 add 0.5ml of culture 2
- Incubate new cultures (step 5 & 6) at 37 degrees for 18 hours (starting time 18:00)
Day 3 (15-9-2012)
Experiment:
- Stop incubation of cultures after 18 hours of growth (at 12:00), cultures should now be well into the stationary faze.
- From all 8 cultures take 1.5 ml sample and put on ice (-20 C) these are negative control reference points (IPTG- and ARA-)
- To 3 of 4 cultures) (day 2 – step 5) each add 100mM IPTG, these will become the IPTGex30, IPTGex60, IPTGex120 cultures
- To 3 of 4 cultures (day 2 – step 6) each add excessive amount of Arabinose, these will become the ARAex30, ARAex60, ARAex120 cultures
- From all 8 cultures (IPTG-, IPTGex30, IPTGex60, IPTGex120, ARA-, ARAex30, ARAex60, ARAex120) take 1.5 ml sample and put on ice (-20 C) (IPTG t0 & ARA t0)
- At 30 min take for all 8 cultures (IPTG-, IPTGex30, IPTGex60, IPTGex120, ARA-, ARAex30, ARAex60, ARAex120) a 1.5 ml sample and put on ice (-20 C) (IPTG t30 & ARA t30)
- Afterwards wash 1 (IPTGex30) of the 3 cultures from step 3 and also from 1 (ARAex30) of 3 of the cultures from step 4 according to the following washing procedure (approx. 30 min):
- Centrifuge at 4000 RMP for 10 min (minimalize cell death at low RPM)
- Remove supernatant
- Resuspendent pellet in 45.5ml LB
- Repeat steps a through c 4 times.
- Put washed and resuspended IPTGex30 and ARAex30 cultures from step 7 into 2 clean (don’t think they have to be sterile) 500ml flasks.
- At 60 min take for all 8 cultures (IPTG-, IPTGex30, IPTGex60, IPTGex120, ARA-, ARAex30, ARAex60, ARAex120) a 1.5 ml sample and put on ice (-20 C) (IPTG t60 & ARA t60)
- Immediately after step 9, repeat steps 7&8 for IPTGex60 & ARAex60 cultures (approx 30 min). Note that the resuspenention during the washing (step 7) should be done in 44ml of LB instead of 45.5ml!
- At 90 min take for all 8 cultures (IPTG-, IPTGex30, IPTGex60, IPTGex120, ARA-, ARAex30, ARAex60, ARAex120) a 1.5 ml sample and put on ice (-20 C) (IPTG t90 & ARA t90)
- At 120 min take for all 8 cultures (IPTG-, IPTGex30, IPTGex60, IPTGex120, ARA-, ARAex30, ARAex60, ARAex120) a 1.5 ml sample and put on ice (-20 C) (IPTG t120 & ARA t120)
- Immediately after step 12, repeat washing procedure of step 7&8 for IPTGex120 & ARAex120 cultures (approx 30 min). Note that the resuspenention during the washing (step 7) should be done in 41ml of LB instead of 45.5ml!
- At 150 min take for all 8 cultures (IPTG-, IPTGex30, IPTGex60, IPTGex120, ARA-, ARAex30, ARAex60, ARAex120) a 1.5 ml sample and put on ice (-20 C) (IPTG t150 & ARA t150)
- Repeat step 14 for every next 30 min until out of time (t180, t210, t240, t270, t300, enz)
Long time exposure protocol
Day 1
- End of the day: Prepare and incubate (37C) 6x 3ml pSB1AT3+LacH containing bacterial cultures (with appropriate antibiotic)
- End of the day: Prepare and incubate (37C) 6x 3ml pSB1AT3+pBAD containing bacterial cultures (with appropriate antibiotic)
Day 2
- Start of the day: To 3 cultures from Day 1, Step 1 add to each 15 ul of 1M IPTG solution
- Start of the day: To 3 cultures from Day 1, Step 2 add to each 120 ul of 100% (1g/ml) Arabinose solution
- Keep all cultures incubated at 37C
Day 3
- Start of day: From 1 of the cultures from Day 2, Step 1 take 2x a 1.65ml sample (1 for miniprep 1 for western blot), discard rest of culture (IPTG+ 1 day exposure)
- Start of day: From 1 of the cultures from Day 2, Step 2 take 2x a 1.65ml sample (1 for miniprep 1 for western blot), discard rest of culture (IPTG- 1 day negative control)
- Start of day: From 1 of the cultures from Day 1, Step 1 (and not used in Day 2, Step 1) take 2x a 1.65ml sample (1 for miniprep 1 for western blot), discard rest of culture (ARA+ 1 day
exposure)
- Start of day: From 1 of the cultures from Day 1, Step 2 (and not used in Day 2, Step 2) take 2x a 1.65ml sample (1 for miniprep 1 for western blot), discard rest of culture (ARA- 1 day
negative control)
- Start of day: To the 2 remaining cultures of Day 2, Step 2 each add 120 ul of 100% (1g/ml) Arabinose solution
- Keep all (remaining) cultures incubated at 37C
Day 4
- Start of day: From 1 of the (remaining) cultures from Day 2, Step 1 take 2x a 1.65ml sample (1 for miniprep 1 for western blot), discard rest of culture (IPTG+ 2days exposure)
- Start of day: From 1 of the (remaining) cultures from Day 2, Step 2 take 2x a 1.65ml sample (1 for miniprep 1 for western blot), discard rest of culture (IPTG- 2 days negative control)
- Start of day: From 1 of the (remaining) cultures from Day 1, Step 1 (and not used in Day 2, Step 1) take 2x a 1.65ml sample (1 for miniprep 1 for western blot), discard rest of culture (ARA+ 2 days exposure)
- Start of day: From 1 of the (remaining) cultures from Day 1, Step 2 (and not used in Day 2, Step 2) take 2x a 1.65ml sample (1 for miniprep 1 for western blot), discard rest of culture (ARA- 2 days negative control)
- Start of day: To the 1 remaining culture of Day 2, Step 2 add 120 ul of 100% (1g/ml) Arabinose solution
- Keep all (remaining) cultures incubated at 37C
Day 5
- Start of day: From the 1 remaining culture from Day 2, Step 1 take 2x a 1.65ml sample (1 for miniprep 1 for western blot), discard rest of culture (IPTG+ 3 days exposure)
- Start of day: From the 1 remaining culture from Day 2, Step 2 take 2x a 1.65ml sample (1 for miniprep 1 for western blot), discard rest of culture (IPTG- 3 days negative control)
- Start of day: From the 1 remaining culture from Day 1, Step 1 (and not used in Day 2, Step 1) take 2x a 1.65ml sample (1 for miniprep 1 for western blot), discard rest of culture (ARA+ 3 days exposure)
- Start of day: From the 1 remaining culture from Day 1, Step 2 (and not used in Day 2, Step 2) take 2x a 1.65ml sample (1 for miniprep 1 for western blot), discard rest of culture (ARA- 3 days negative control)
Experimental setup for consistent testing of IPTG+/- pSB1AT3+MTase containing bacteria in different growth phases (Log and Stationary)
Step 1 – Growth Curve Measurement
Growthcuves should be measured in for the pSB1AT3 + Mtase construct containing bacteria. This is critical because all tests should be performed on plasmids extracted from cultures that are either in the log (exponential) phase or fully grown cultures. Bought conditions should be used for bought IPTG+ and IPTG- cultures.
Setup
Starting media:
- 100ml LB
- 100ml LB containing 100milliM IPTG
Starting bacteria:
- 2x 10ul pSB1AT3 + MTase containing bacteria form overnight (stationary phase) culture
Other requirements:
- 1ml cuvette accepting spectrophotometer
- 44x 1ml cuvette
- 1x 1ml Demi or MilliQ containing cuvette (for calibration)
Experiment
- 1.Add 10ul pSB1AT3 + MTase containing bacteria from overnight (stationary phase) culture to 100ml LB and to a 100ml LB containing 100milliM IPTG media
- 2.Incubate at 37 C in shaker
- 3.Measure OD of both cultures every 30 min for 10 hours
- 4.After 10h leave cultures in 37C shaker
- 5.Next day measure OD again for bought cultures, and 30 min later (to confirm that the culture is actually stationary at this time)
Expectation
Literature suggests that (if there is enough medium available) the culture will be in the log fase after 1h till about 6h.
Experience suggests that 10ml cultures will be in stationary phase after 16h of 37C growth (overnight cultures)
Step 2 – Stationary phase Positive (IPTG+) and Negative (IPTG-) controls
These experiments will tell us if the leakiness of the promoter is enough to cause all sites to be methylated. And will (in conjunction with the results from step 3) later tell us something the influence of growth rate on the equilibrium between methylation loss (due to plasmid replication and death) and gain (due to leakiness of the promoter).
Setup
Starting media:
- 10ml LB
- 10ml LB containing 100milliM IPTG
Starting bacteria:
- 2x 10ul pSB1AT3 + MTase containing bacteria form overnight (stationary fase) culture
Other requirements:
- Miniprep kit
- Nanodrop machine
- ScaI restriction enzyme
- EcoRI restriction enzyme
- Gels
- Standard ladder
Experiment
- 1.Add 10ul pSB1AT3 + MTase containing bacteria from overnight (stationary phase) culture to 10ml LB and to a 10ml LB containing 100milliM IPTG media
- 2.Put cultures in shaker and incubate at 37C
- 3.Wait till cultures are in stationary phase, exact time is determined in step 1 (experience suggests that this is after 16h which means an overnight culture in practice)
- 4.Do a plasmid extraction from bought cultures using miniprep, note however that a little bit of both cultures (10ul) should be preserved for step 3!
- 5.Measure DNA yields from both extractions using nanodrop
- 6.Dilute or concentrate until DNA concentration for both extractions is the same (aim for 1000ug/ul *Guess*)
- 7.Do standard 1h digestions of both DNA extracts in the following variations ScaI, ScaI + EcoRI, EcoRI, None.
- 8.Run DNA from both cultures for all variations on gel, with the ladder on both sides and in the middle. Exact layout: **Ladder, IPTG- DNA digested by ScaI, IPTG- DNA digested by ScaI + EcoRI, IPTG- DNA digested by EcoRI, IPTG- DNA digested by None, Ladder, IPTG+ DNA digested by ScaI, IPTG+ DNA digested by ScaI + EcoRI, IPTG+ DNA digested by EcoRI, IPTG+ DNA digested by None, Ladder.
Expectation
Ideal results would be that the Positive control (IPTG+) will result in intensity shift to the uncut/1cut plasmid band in the ScaI digestion and a shift to the top band (plasmid only cut by EcoRI) in the ScaI + EcoRI digestion. Whereas for the Negative control (IPTG-) this shift would be the opposite. Pilot experiments already suggest that this shift of the Negative control is not as severe as would be desired.
Might we find that the Positive and Negative control of this experiment are similar and this result is more like what we would like to see from the positive control it would indicate that in the case of a static culture leaky expression is enough to methylate all the sites.
Seeing similar results but no clear shift (similar to the pilot experiment) will indicate that all sites are possibly methylated but that ScaI have a small chance of cutting methylated sites.
Step 3 – Log phase Positive (IPTG+) and Negative (IPTG-) controls
This experiment is done in order to determine the effect that bacterial growth has on the loss and gain of methylated sites and the equilibrium it might establish.
Setup
Starting media (much more than in step 3 because less DNA will be extracted per ml due to lower growth time):
- 100ml LB
- 100ml LB containing 100milliM IPTG
Starting bacteria:
- 10ul pSB1AT3 + MTase containing bacteria form step 2.4 (stationary phase) IPTG+ culture
- 10ul pSB1AT3 + MTase containing bacteria form step 2.4 (stationary phase) IPTG- culture
Other requirements:
- Miniprep kit
- Nanodrop machine
- ScaI restriction enzyme
- EcoRI restriction enzyme
- Gels
- Standard ladder
Experiment
- 1.Add 10ul pSB1AT3 + MTase containing bacteria from step 2.4 (stationary phase) IPTG+ culture to the 100ml LB containing 100milliM IPTG media
- 2.Add 10ul pSB1AT3 + MTase containing bacteria from step 2.4 (stationary phase) IPTG- culture to the 100ml LB media
- 3.Put cultures in shaker and incubate at 37C
- 4.Wait till cultures are in log phase, and let them grow till near the end of the log phase, exact time is determined in step 1 (literature suggests that this is after 6h which means that this can be on the same day as you do step 2.4)
- 5.Do a plasmid extraction from bought cultures using miniprep
- 6.Measure DNA yields from both extractions using nanodrop
- 7.Dilute or concentrate until DNA concentration for both extractions is the same (aim for 1000ug/ul *Guess*). To achieve the desired DNA concentration step 5 and 6 may need to be repeated, this is also the reason why the cultures in step 3 needed to be bigger.
- 8.Do standard 1h digestions of both DNA extracts in the following variations ScaI, ScaI + EcoRI, EcoRI, None.
- 9.Run DNA from both cultures for all variations on gel, with the ladder on both sides and in the middle. Exact layout: Ladder, IPTG- DNA digested by ScaI, IPTG- DNA digested by ScaI + EcoRI, IPTG- DNA digested by EcoRI, IPTG- DNA digested by None, Ladder, IPTG+ DNA digested by ScaI, IPTG+ DNA digested by ScaI + EcoRI, IPTG+ DNA digested by EcoRI, IPTG+ DNA digested by None, Ladder.
Expectation
In the ideal case the Positive result (IPTG+) would only show the uncut/1 cut plasmid band in the ScaI digestion and only the top band (uncut plasmid) in the ScaI + EcoRI digestion. And the opposite results in the negative control (IPTG-)
If similar results to the expected shift in step 2 are observed than the growth rate of the culture doesn’t have any impact on the methylation gain and methylation loss equilibrium.
If similar results to step 2 but no shift is observer this means that ScaI is able to cut a certain methylated sites in some with a low affinity and that all sites are methylated.
Possible Step – Testing under different concentrations of IPTG
Repeat Step 2 and 3 using a different concentrations of IPTG. If step 1 suggest that IPTG influences the growth rate of the bacteria significantly also redo step 1 with the different concentrations of IPTG.
Possible Step – Testing different concentrations or exposure times of/to ScaI
If ScaI is found to be able to sporadically cut methylated sites its useful to find out how sporadic this occurs. This can be done by repeating steps 2 or/and 3 using lower concentrations of ScaI or varying the exposure time (normally 1h) to the restriction enzyme. Note however when varying the exposure times to ScaI you need to perform the EcoRI digestion first and separately.
 "
"

