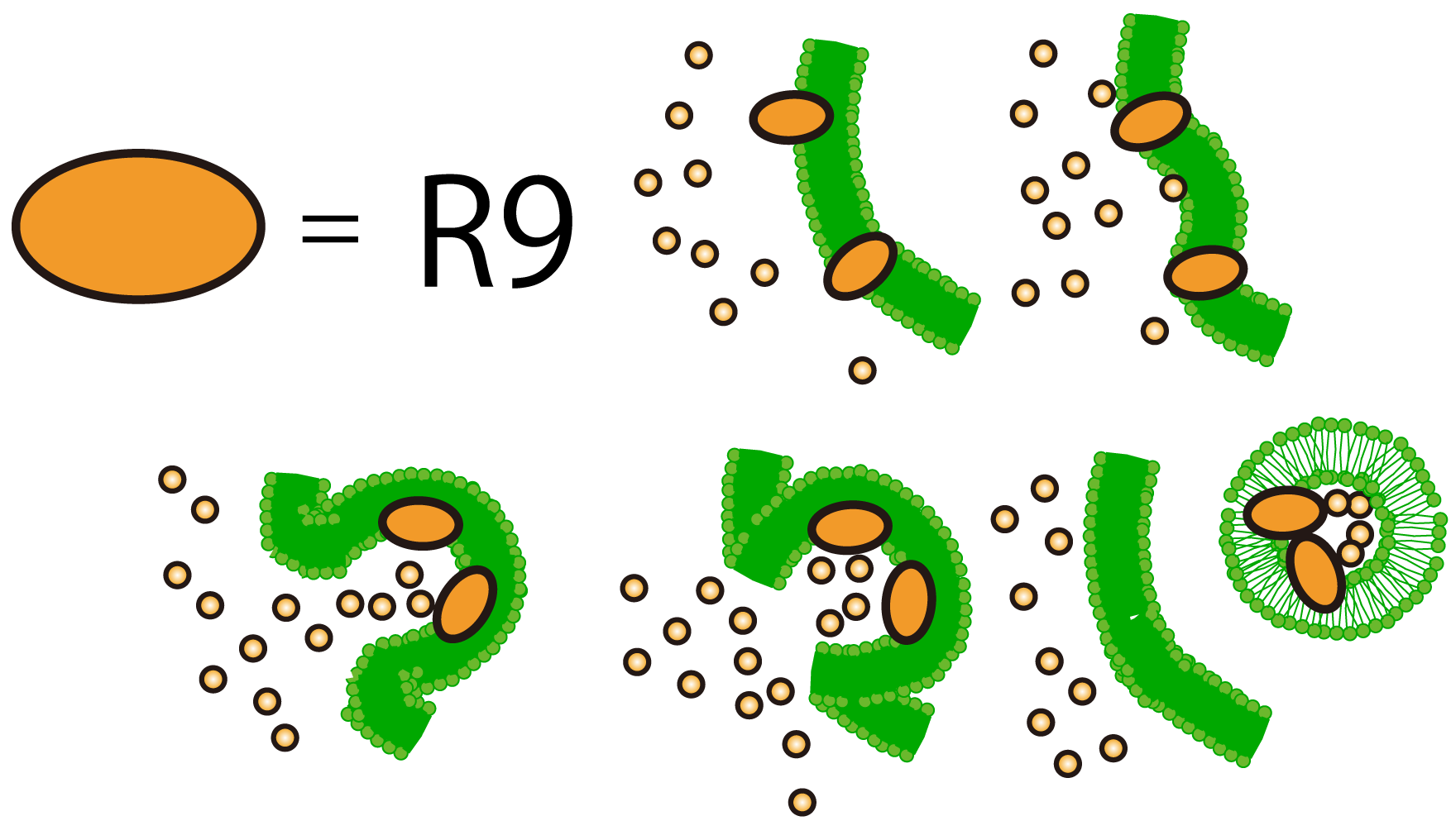Team:Kyoto/Project
From 2012.igem.org
Contents |
Have you ever seen flower fairies? Probably no(some of you might come across them in your childhood), because they are imaginary creatures existing only in fairy tales. What if we can live with flower fairies? Their lovely powers to make flowers bloom would be profitable for us, including application to agriculture. That’s why we set our project to realize it with synthetic biology, Flower Fairy E.coli!
Our goal is to produce E.coli which can make flowers bloom as Flower Fairies. To make it possible, we focus on FT protein, the identity of Florigen.
There are four issues in order to create Flower Fairy E.coli. It is unclear whether E.coli(prokaryote) could produce functional FT properly because FT is usually produced in the plant cells(eukaryote). After produced, FT have to go through four walls, inner and outer membranes, a cell wall of the plant cells and a cell membrane of the plant. Even though FT could get inside of the cells, it is unknown whether FT protein transcribed in E.coli can activate shoot apex cells and bloom flowers.
We have to go through four steps for purpose of obtaining our goal-Flowering Fairy E.coli-
The four steps are composed of “EXPRESSION”,”SECRETION”,
”PENETRATION”, and ”ACTIVATION”
1.EXPRESSION
On the first step; EXPRESSION, E.coli produce florigen inside it.
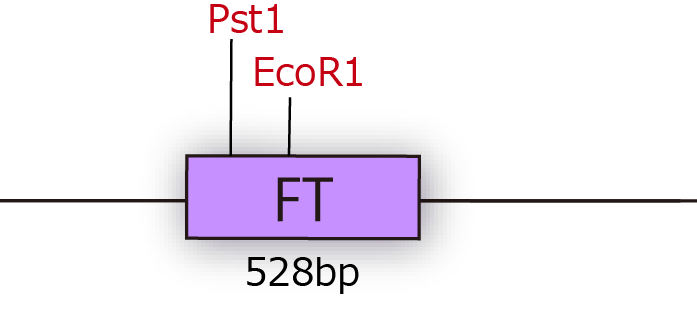
Professor Araki kindly gave us FT cDNA of Arabidopsis thaliana in TOPO blunt end 2(Invitrogen). When we got FT gene, we had a difficulty in constructing iGEM parts. The first problem is that FT sequence had two cleavage sites of iGEM restriction enzymes. In order to eliminate cleavage sites of iGEM restriction enzymes, we performed Inverse PCR of plasmids with two kinds of primers which contain one base mis-match between primer and cDNA. Inverse PCR is a kind of PCR with the use of restrose primers. This PCR enables us to mutate FT because tensile direction is reverse.
Of which 3’ ends get longer in the direction of the outside of reproduction domain in contradiction to nomal PCR. We succeeded in mutating FT plansmid by this PCR method.
As a result, we could get mutated plasmids, which are not cleaved by iGEM restriction enzymes. In this way, we made FT gene available(Fig.1-2)
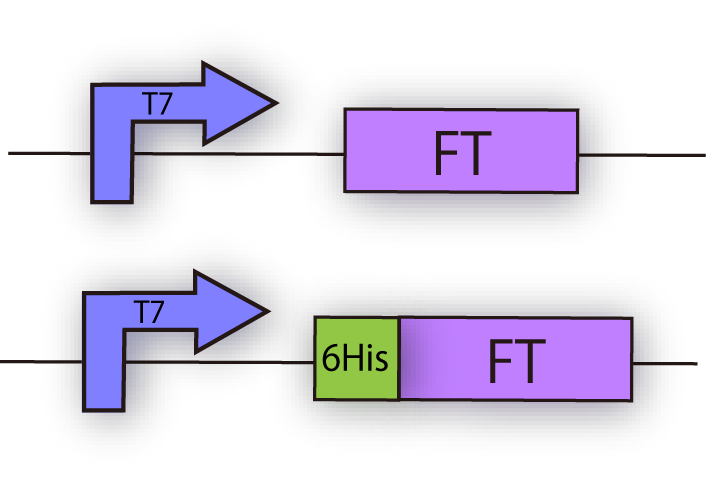
We constructed the plasmid shown in the Fig.1-3. We wanted to maximize the expression level of FT. T7 promoter is the strongest promoter in terms of FT expression, so we use T7 promoter. And we wanted to purify FT protein in order to take an experiment of FT penetration into plant cell membranes. 6 His tag enables us to protein purification from E.coli.
FT and His tagged FT are regulated by T7 promoter, BBa_I719005 and strong RBS, BBa_B0034.
In order to confirm the expression of FT protein, we performed western blotting using anti-FT goat antibody and checked the place of the FT protein band.
As a result, FT and 6 His:FT bands were observed at the expected molecular weight region. We succeeded in expression of FT and 6 His::FT proteins in E.coli.
We cooperated with KAIT-Japan and the mark on the left indicates Biosafety Level of our parts.
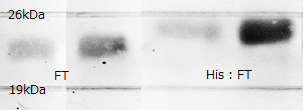
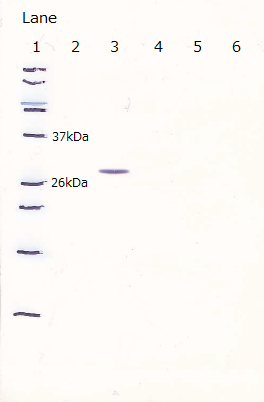
Each plasmid shown in Fig.1-3 was transformed into BL21(DE3). Cells were precultured overnight and diluted into fresh SOC medium. IPTG was added when OD600 was approx. 0.5, then cells were incubated for 4h at 20°C. 100µL of culture was used for SDS-PAGE.
Lane1 : FT Cell lysate, not induced
Lane2 : FT Cell lysate, IPTG induced
Lane3 : His:FT Cell lysate, not induced
Lane4 : His:FT Cell lysate, IPTG induced
2.SECRETION
On the second step; SECRETION, E.coli secretes florigen outside of the cell.
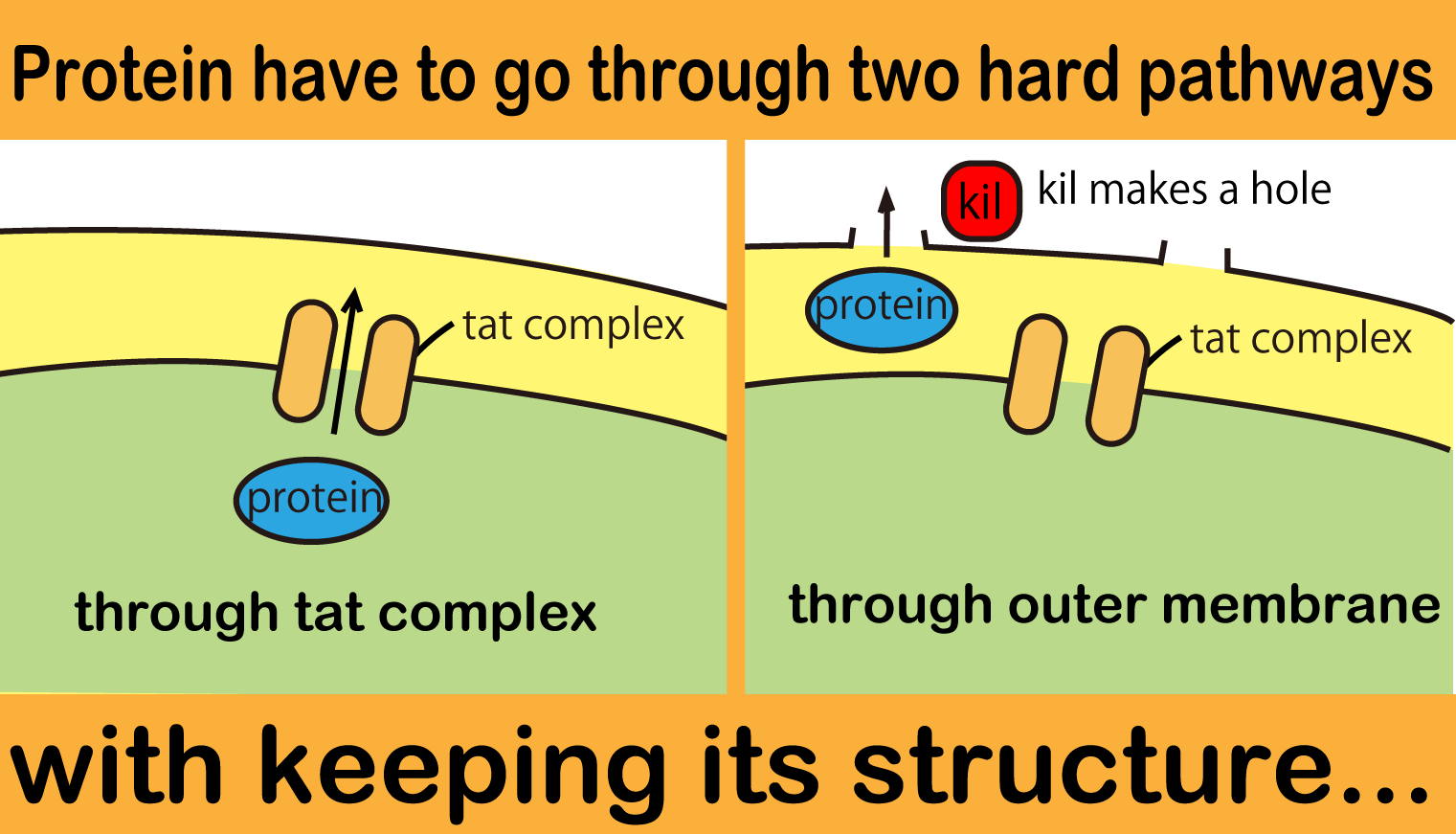
Even though our E.coli can produce FT protein, a big issue remains: how they can transport proteins to the outside of the cells? To make it possible, we tried to make Tat cassette and kil protein generater. This cassette makes E.coli carry proteins with TorA signal via Tat protein transportation pathway from the cytoplasm to the periplasm. The periplasm is a space between inner and outer membrane. Kil protein encourages proteins to move from periplasm to outside[8]. We made this protein secretion system and visualized and confirmed its function by using GFP.
Result 1: Modified TorA signal
The Twin Arginate Translocation pathway(Tat) is one of the secretion systems E.coli originally has. This system can carry proteins that have TorA signal at N terminal. TatA, TatB and TatC compose Tat complex on inner membrane. Tat complex recognizes TorA signal peptide and then it transports proteins (with TorA) from cytoplasm to periplasm with maintaining their folding. In short, proteins secreted via Tat pathway can keep active.
In this experiment, we wanted to design an applicable TorA signal device to meet various needs and to check the function of signal sequence. TorA signal was, actually,submitted by Canbrige 2011(BBa_K233307) cause a stop codon between signal peptide and target coding sequence(CDS) when you assemble them by standard or 3A assembly. For these reasons, all of other teams make an effort to combine TorA signal to targets, such as using Gibson assembly. That's too trouble!
To avoid appearing a stop codon in scar sequence between TorA signal and a target protein CDS, we made Frameshift mutation twice on TorA signal. As a result, you can make TorA-fusion target protein by standard or 3A assembly. In addition to that, our TorA signal has RBS. This can be an easy-to-use biobrick part. It can shorten the time of constructions because it only needs a promoter and a target protein.
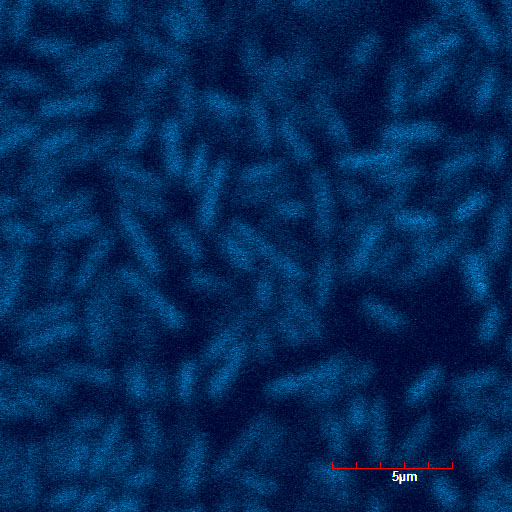
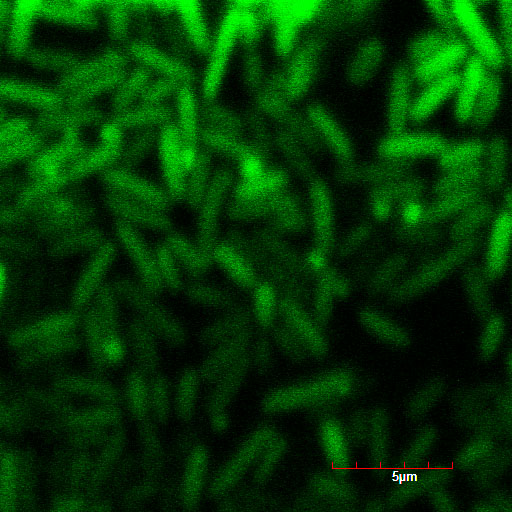
We read the sequence data of our modified TorA signal and confirmed stop codon doesn't appear when it used in Standard or 3A assembly. Using green fluorescent protein (GFP) as a target protein, we observed the TorA-GFP fusion-expressing cells(Fig.1-1,1-2). The TorA-GFP fusion was successfully expressed. This means RBS in our TorA signal worked.
Result 2: evaluation of kil protein
In previous iGEM competitions, some kinds of parts and devices for protein translocation were already developed. One of the most widely used parts is lysis cassette. This part causes cell lysis and, as a result, makes E.coli scatter materials it includes. This style is, unfortunately, not suitable for our project because of the possibility of accidental all-death. Generally speaking, the concentration of E.coli on a flower is not high so that it can’t be ignored that the possibility of occurring all cell-death. Once all our Fairies disappear, a flower wouldn’t bloom. In addition to that, lysising E.coli is NOT CUTE.
Kil makes holes in outer membrane and we expect that a protein goes through these holes. Larger hole area means larger amount of proteins can go out. On the other hand, the function of outer membrane is essential for E.coli to survive. Overexpression of Kil,threfore, causes cell death. For this reason, we must check whether our kil gene is overexpressed or not.
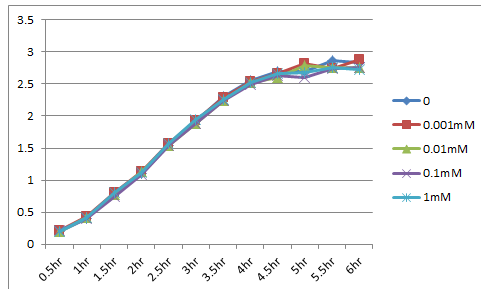
We made the construct, lacp-RBS-Kil-double terminator, whose backbone is pSB3C5. After culturing for 18hr at 37℃, we eliminated the supernatant using a centrifuge, and diluted it until OD600=0.1. Then we dispensed it. The dispense volume was 3mL. We added 0/0.001/0.01/0.1/1mM IPTG to each. While culturing again at 37℃, we measured OD600. The table below shows the results. This result indicates that the expression of LacP-RBS-Kil-DT(pSB3C5) makes no effect on survival of E.coli.
Result 3: Construction of Tat cassette
TatABC composes a pathway from cytoplasm to perip Detail of Our Secretion System
Tat secretion cassette with constitutive promoter(BBa_K797004)
Our secretion system is constructed by TatABCD, Kil and another gene. Another gene is PspA (phage-shock protein A) gene and E.coli has it originally. This gene is expressed when their inner membrane is damaged. PspA maintains membrane potential and H+ concentration gradient between periplasm and cytoplasm.
Our secretion system makes many holes in inner and outer membranes. In other words, E.coli which has our secretion system is under the membrane stress conditions. But by introducing pspA into our Flower Fairy E.coli, the E.coli comes to be able to maintain the vitality, though they have many holes in the membrane.
This cassette allows E.coli to secrete proteins with TorA signal. Wild type Tat protein secretion system is so weak that Kyoto 2012 constructs Tat cassette to reinforce the ability of transportation of Tat system. This part includes TatA, B and C proteins coding region and pspA (phage shock protein A). Tat A, B and C proteins are the main components of Tat complex where proteins with TorA signal go through, and pspA can encourage protein secretion via Tat system. Kyoto 2012 suggests this new way of secretion and provides iGEMers with this cassette regulated by constitutive promoter.
We checked the sequence of TatABCD(BBa_K797000) and the sequence of pspA (BBa_K797001) individually, and then, we made Tat construction composed of constitutive promoter(BBa_J23107), TatABCD
(BBa_K797000),pspA(BBa_K797001) and double terminator
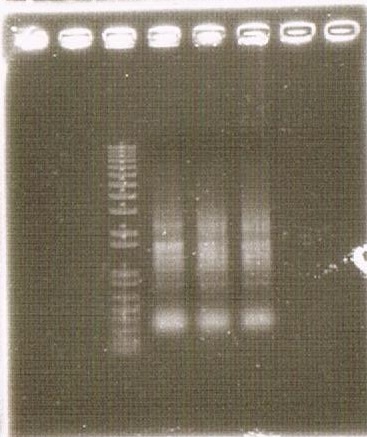
(BBa_B0015). This TAT secretion cassette is too long device to sequence, so that we performed electrophoresis of this cassette and confirmed the length of our parts.
3.PENETRATION
1. Cool system for penetration --R9 peptide--
On the third step; PENETRATION.
FT protein from Flower Fairy E.coli needs to enter into plant cells in order to induce plants to bloom.
From early stage, we sought various ways to penetrate FT protein into plants, but each way has serious problems.
In such a situation, we found the way of penetration into cells using polyarginine. This is called R9 peptide, a type of Cell Penetrating Peptide (CPP). R9 peptide consists of nine arginine residues.
R9 peptide is thought to act on a cell membrane and causes macropinocytosis,a specific form of endocytosis. The use of CPP in plant cells is already verified.
R9 peptide adheres to cell membrane of plants because of hydrophobic character.The cell responses to the stimulus and cause macropinocytosis. FT protein around an invaginating region of the cell is taken in the cell.
Plants by themselves practice CPP to transport biomolecules such as proteins inside the cell, in spite of their cell walls.
Then, we determined to cause penetration of FT protein by R9 peptide.
2. GFP antibody specificity check
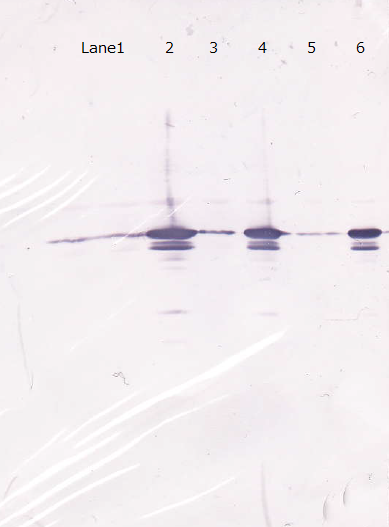
Lane1 : Cell lysate 10µL, not induced
Lane2 : Cell lysate 10µL, IPTG induced
Lane3 : Cell lysate 5µL, not induced
Lane4 : Cell lysate 5µL, IPTG induced
Lane5 : Cell lysate 2µL, not induced
Lane6 : Cell lysate 2µL, IPTG induced
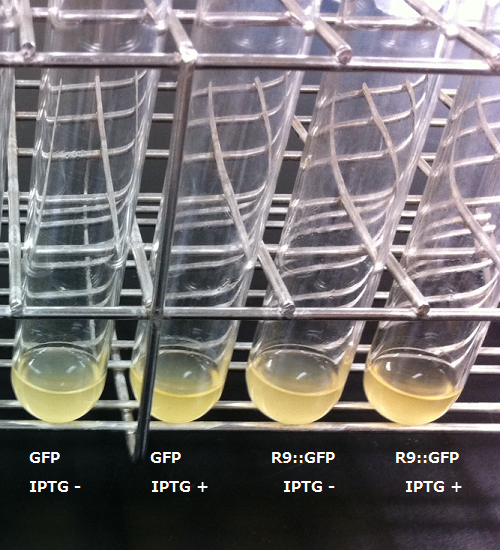
First, we checked the specificity of anti GFP monoclonal antibody against R9::GFP. We wanted membrane to penetrate GFP in order to confirm R9 peptide system by fluorecence. This is because we didn't know whether our E.coli expressed R9 peptide fusioned GFP properly.
We used the existing GFP generator parts, [http://partsregistry.org/Part:BBa_I746915 BBa_I746915].
The parts is consist of T7 promoter 6-his tagged superfolder GFP.
Unfortunately, we used inappropriate molecular marker and could'nt confirm the molecular weights of samples.
Each samples induced with IPTG showed one main band and one extra band, and uninduced controls showed one bands.
The relation of intensity of bands between induced samples and controls is corresponded to the existence of IPTG.
Minor bands of induced samples are considered as the degradated GFP.
From this result, we concluded that GFP antibody has enough specificity.
After the 4h of IPTG induction, we noticed that E.coli expressing R9::GFP were growing poorly.
Moreover, we couldn't get any bands of R9::GFP, as shown in the Fig.3-4.
We suspected that R9:GFP is not transcripted in mRNA, so we did RT-PCR using GFP specific primers.
GAPDH is used as internal control.
As a result, we concluded that R9-GFP mRNA is expressed, but not translated in proteins, perhaps because of its cytotoxicity.
3. Separating R9 peptide and GFP
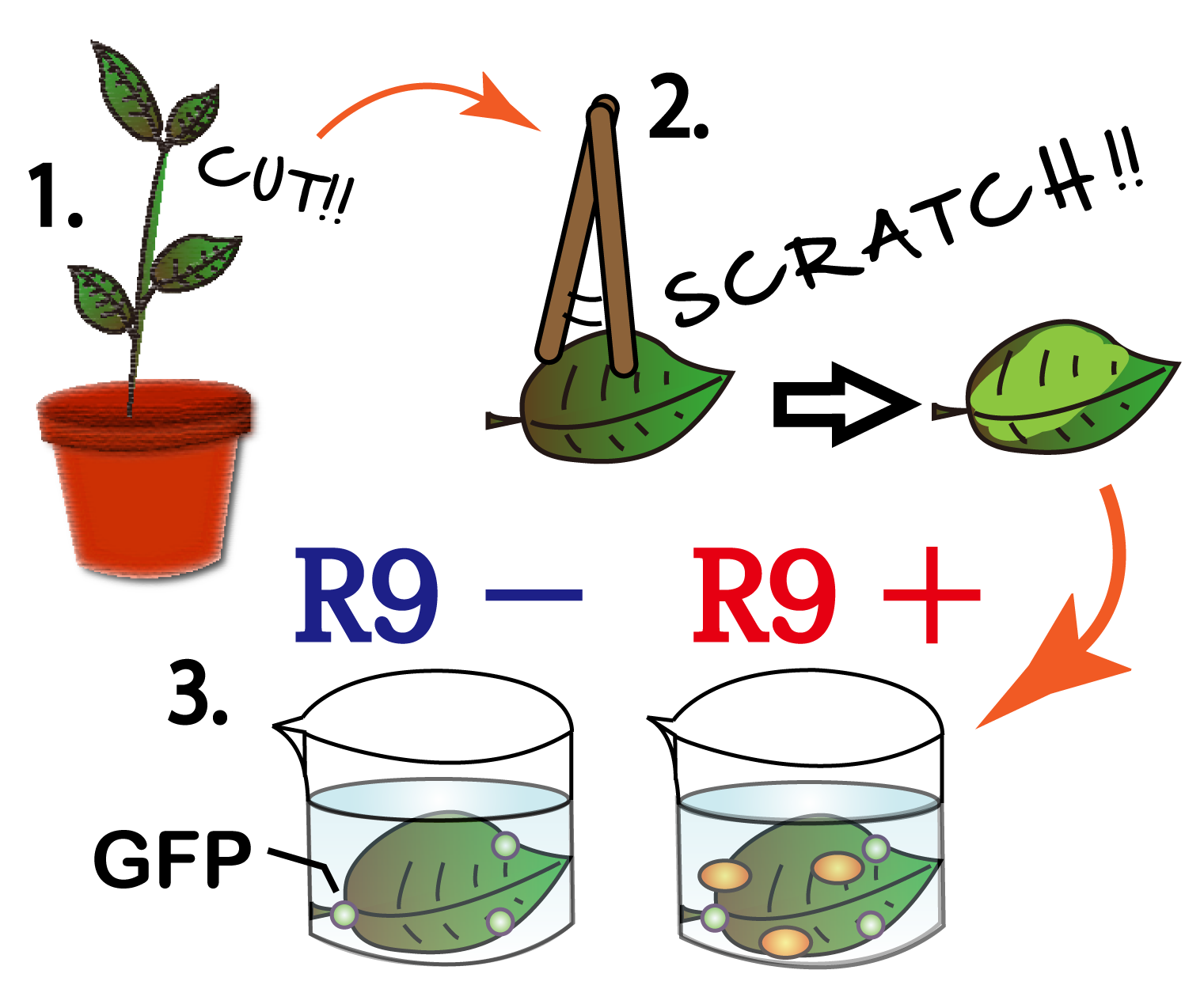
When R9 and GFP were connected, E.coli can't produce R9 fuged protein. Then for the purpose of the function of R9, we compared between R9+ and R9-. We scratched the cuticule of plant cells and soaked them into a sollution of GFP and R9 or only GEP. This GFP protein was purified with 6His tag. After 5 minutes we washed cells by PBS in order to wash GFP and R9peptide away. Then we succeeded in getting the figure of GFP fluoresence.
The controls on the left were soaked in only GFP, and the samples on the right-hand side were soaked in GFP and R9. These two pictures indicate the action of R9 peptide. R9 peptide kept GFP in or around plant cells. This figure strongly suggests that R9 peptide works successfully and penetrates cell membrane with GFP.
Fig. Verification of R9 function with use of GFP.
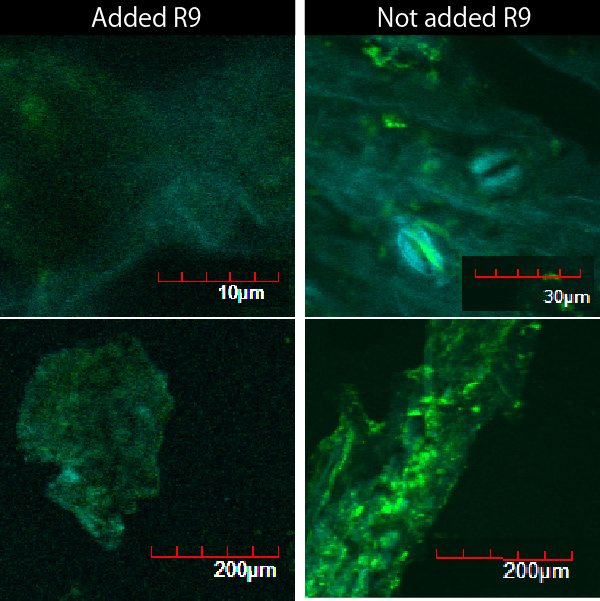
These pictures shows cells of Arabidopsis thaliana leaves soaked in a solution for five minutes, and Hoechst dyeing. Left side samples are soaked in only GFP, the right side samples in GFP and R9 peptide. From top to bottom, nuclei by Hoechst (10 times magnification), GFP fluorescence (10 times), nuclei by Hoechst (60 times), GFP fluorescence (60 times) (With confocal microscope Fluoview FU10i OLYMPUS)
4. Activation
On the final step; Activation. We verified whether FT normally worked in plant cells.
FT protein is derived from plant cells and it is capable of post-translational modification. E.coli cannot do post-translational modification, so FT protein derived from Flower Fairy E.coli may not work normally. As a final step, we tried to confirm whether FT protein by our E.coli led to flower formation.
How to Verify FT Function
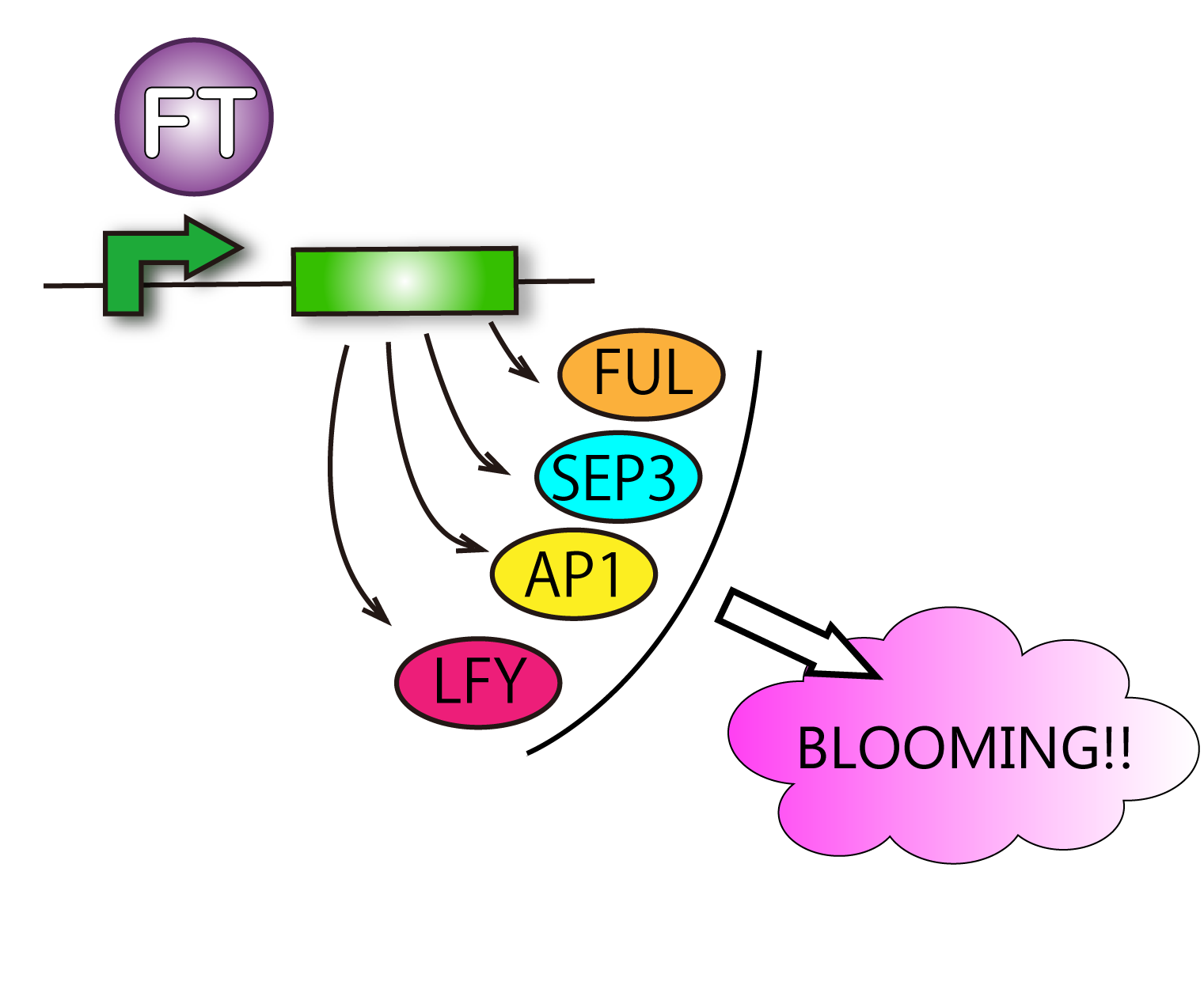
FT protein increases transcriptive activity of several proteins which lead to flower formation. For that reason it can be said that we have verified FT function when we have found rises of activities of the proteins.
Although such proteins activated by FT are various, we check APETALA 1(AP1), SEPALLATA3(SEP3) and FRUITFULL(FUL). This is because AP1 is the representative protein activated by FT, and SEP3 and FUL are activated in leaves. It is difficult for us to handle cells of tips of stems. So we focused on cells of leaves. Leaves' cells are easy to handle for us. We performed RT-PCR in order to investigate FT protein's function.
We performed RT-PCR to compare mRNA expression of Arabidopsis leaves treated with/without FT.
Two types of samples were prepared, one is treated with FT and the other is treated with GFP as a control.
GFP was used as a control because its molecular weight(27kDa) is relatively similar to that of FT(20kDa.)
Fig.4-2 is the result of RT-PCR.

30mg of leaves of Arabidopsis thaliana before bolting were used for one sample. FT or GFP protein and R9 peptide were diluted in PBS (pH7.4), 50ug/L and 500ug/uL each. Leaves were soaked into FT-R9 or GFP-R9 solution for 5min. and incubated for 16hr. in PBS(pH7.4.) After incubation, leaves were freezed with liquid nitrogen and glinded immediately.
Total RNA was extracted by phenol-chloroform extraction. cDNA was synthesized by reverse transcription and used as templates of RT-PCR. TUBULIN was used for internal control of mRNA expression.
Lane1:TUBULIN (GFP-R9 treated) amplicon 61bp
Lane2:TUBULIN (FT-R9 treated)
Lane3:FUL (GFP-R9 treated) amplicon 132bp
Lane4:FUL (FT-R9 treated)
Lane5:SEP3 (GFP-R9 treated) amplicon 87bp
Lane6:SEP3 (FT-R9 treated)
Lane7:AP1 (GFP-R9 treated) amplicon 958bp
From this results, though we could not confirm the function of FT, upregulation of FUL, SEP3, and AP1
but we could amplify each gene successfully.
One possible reason of this failure is the poor quality of extracted RNA in this experiment.
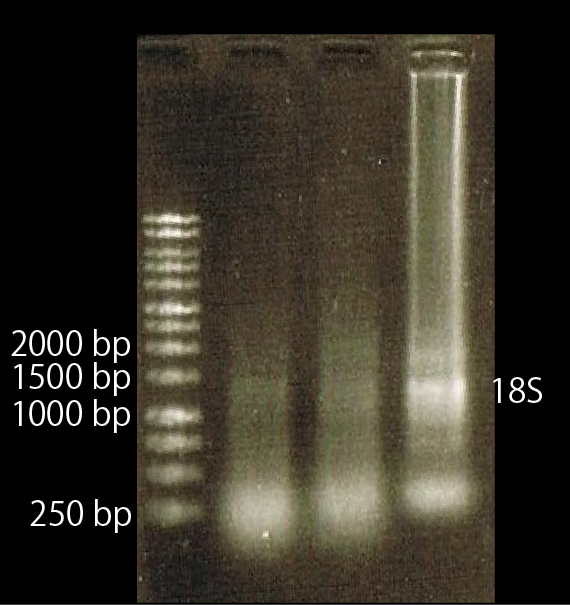
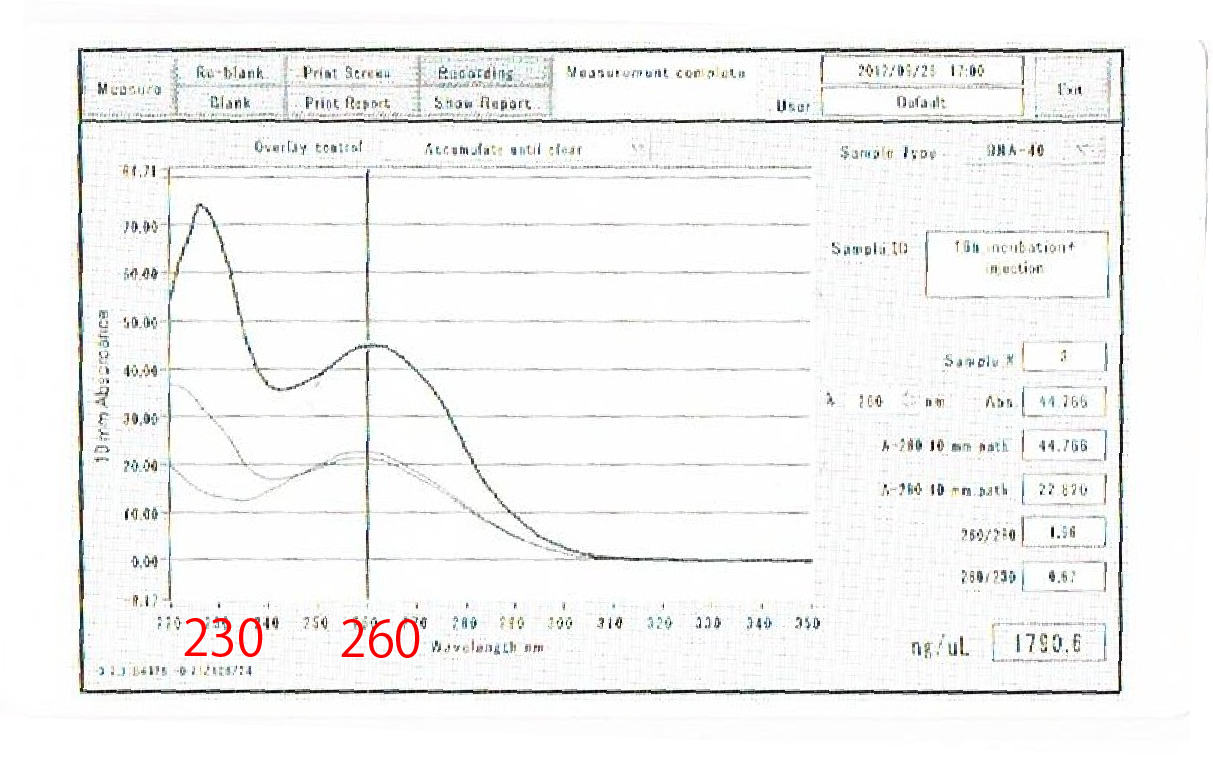
To check this, we compared the total RNA by electrophoresis, shown in fig.4-3.
As shown in the Fig.4-3, RNA samples in this time are degradated.
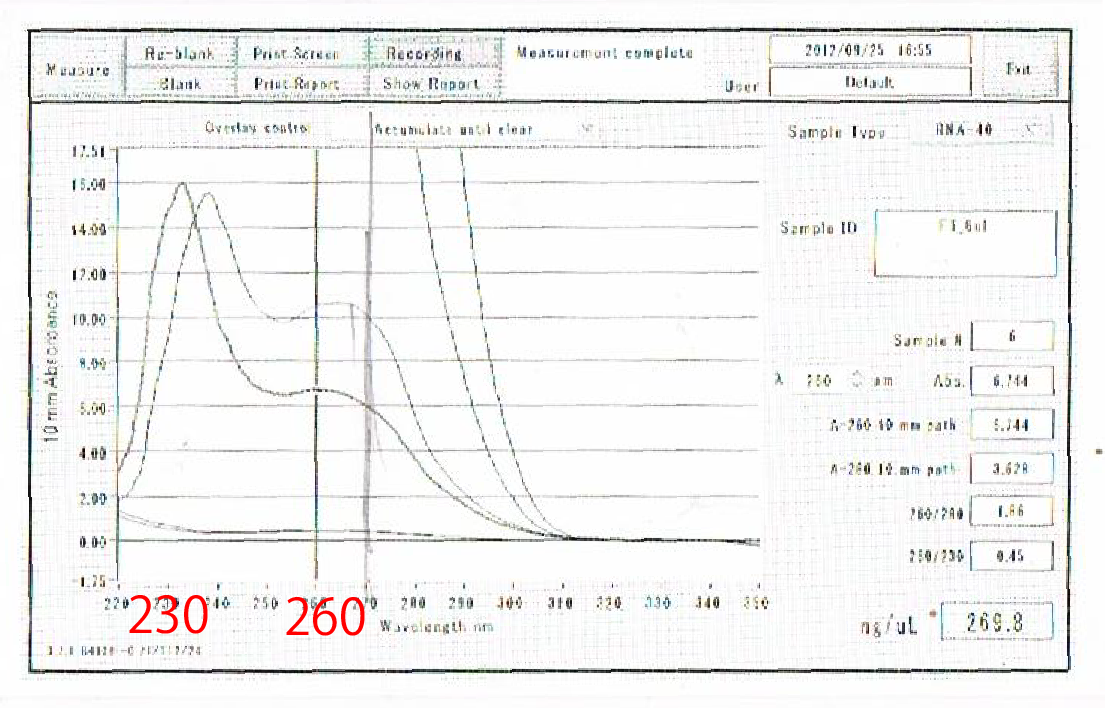
Moreover, the waveforms of them had a law peak at 260nm(Fig.4-4.)
So, it was required to improve the method of RNA exraction.
We improved following things;
1. Total leaf volume was increased.
2. Samples ware freezed with liquid nitrogen and suspended in ISOGEN more rapidly.
3. We centrifuged samples and collectted supernatant twice after adding ISOGEN.
After the improvement, 260nm peak became higher and the degradation is minimized(Fig.4-4, 4-5.)
Now we are trying to check the function of FT.
Achivement
To mutate and standardize FT sequence as a iGEM part.
To confirm expression of FT protein in E.coli.
Future Works
We noticed only flowering and florigen in this time but there are many many other plant hormones. We made translocation pathway from E.coli into plant cells, so we will be able to introduce plant hormones into plant cells if E.coli can make them. It means we can control plant growth in any stage through genetically engineered E.coli. In the future that is not so far, we will be able to meddle in plants' growth――germinating, elongation, flowering, and fructification. We human will finally accomplish a technology that control plants perfectly.
Moreover, R9 peptide functions not only plant cell. R9 peptide works on animal cell similarly. It means that we found a pathway into any kinds of cells. R9 peptide tag enables us to introduce proteins into any cells, so we will be able to controll all living cells using this technology.
Safety
We cooperated with KAIT-Japan and the mark on the left indicates Biosafety Level of our parts.
[1]Microsugar Chang et al. (2005)"Cellular internalization of fluorescent proteins via arginine-rich intracellular delivery peptide in plant cells" Plant Cell Physiol, 46(3), 482–488
[2]Paula Teper-Bamnolker and Alon Samach1 (2005) "The flowering integrator FT regulates SEPALLATA3 and
FRUITFULL accumulation in Arabidopsis leaves" The Plant Cell, 17, 2661–2675
[3]Philip A. Wigge et al. "Integration of spatial and temporal information during floral induction in Arabidopsis
[4]Sara Trabulo et al.(2010). "Cell-penetrating peptides—mechanisms of cellular uptake and generation of delivery
systems" Pharmaceuticals, 3, 961-993
[5]Unnamalai N, Kang BG, Lee. (2004) "Cationic oligopeptide-mediated delivery of dsRNA for post-transcriptional gene silencing in plant cells." FEBS Lett 21;566(1-3):307-10.
[6]Tracy Palmer and Ben C. Berks.(2012) "The twin-arginine translocation (Tat) protein export pathway" Nat Rev Microbiol, 10(7), 483-96
[7]Choi JH, Lee SY.(2004) "Secretory and extracellular production of recombinant proteins using Escherichia coli" Appl Microbiol Biotechnol, 64(5), 625-35
[8]Miksch G, Fiedler E, Dobrowolski P, Friehs K.(1997) "The kil gene of the ColE1 plasmid of Escherichia coli cntrolled by a growth-phase-dependent promoter mediates the secretion of a heterologous periplasmic protein during the stationary phase" Arch Microbiol, 167(2-3), 143-50
[9]Seibel BA, Walsh PJ.(2002) "Trimethylamine oxide accumulation in marine animals: relationship to acylglycerol storage" J Exp Biol, 205(Pt 3), 297-306
[10]Thomas JD, Daniel RA, Errington J, Robinson C.(2001) "Export of active green fluorescent protein to the periplasm by the twin-arginine translocase (Tat) pathway in Escherichia coli." Mol Microbiol, 39(1), 47-53
[11]Galán JE, Collmer A.(1999) "Type III secretion machines: bacterial devices for protein delivery into host cells." Science, 284(5418), 1322-8
[12]Suit JL, Luria SE.(1988) "Expression of the kil gene of the ColE1 plasmid in Escherichia coli Kilr mutants causes release of periplasmic enzymes and of colicin without cell death." J Bacteriol, 170(10), 4963-6
[13]DeLisa MP, Lee P, Palmer T, Georgiou G.(2004) "Phage shock protein PspA of Escherichia coli relieves saturation of protein export via the Tat pathway." J Bacteriol, 186(2), 366-73
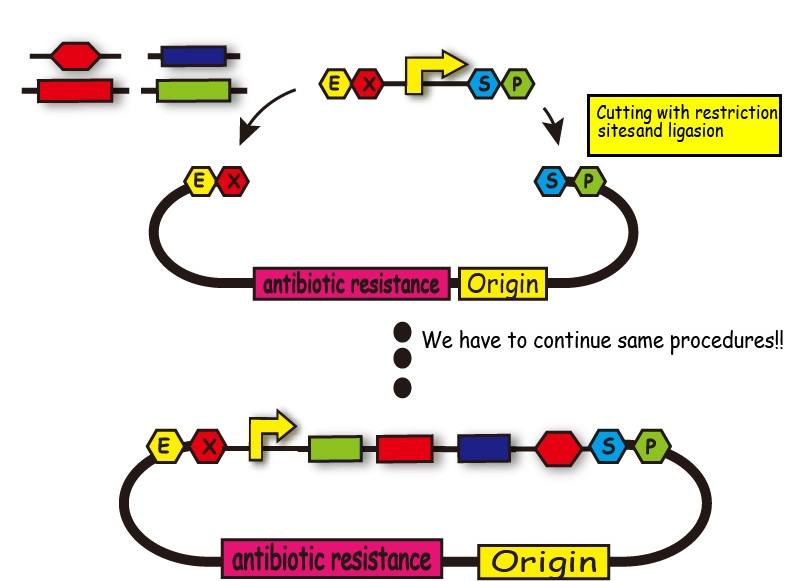
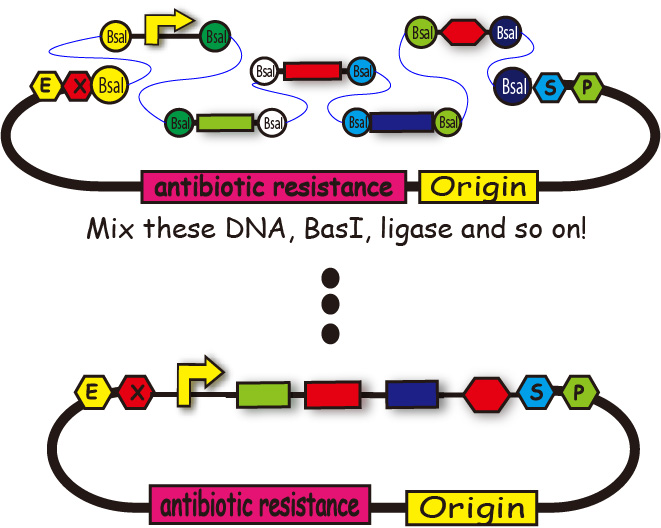
BioBricks are useful for us because we can look for required BioBrick parts from their registory and recombine genes easily. When we want to introduce many parts into one plasmid, however, we have to repeat the process; restrict enzyme digestion and ligation. It takes us too much time and sometimes we lose time for other experiments.
We want to reduce the time required for the recombination of genes and get time for verification of the expression and the effect of genes.
Golden Gate assembly is one of the ways to make it possible.
Some teams like 2011 WHU-China have used this assembly. But they didn't seem to spread Golden Gate Assembly through other iGEM teams. So, we created plasmid backbone parts [http://partsregistry.org/Part:BBa_K797013 "BBa_K797013"] to make it easier to use Golden Gate assembly.
We also created a software which designs primers for Golden Gate assembly.
What's Golden Gate Assembly
Golden Gate Assembly is developed by Carola Engler, Ramona Gruetzner, Romy Kandzia and Sylvestre Marillonnet.[2]
This method enables us to introduce plural gene segments into one plasmid all at once.
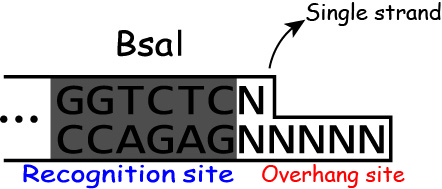
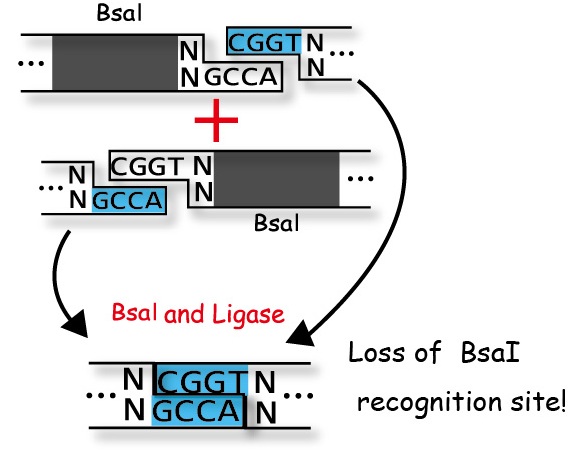
Golden Gate Assembly takes advantage of the characteristic restrict enzyme "BsaI".
Most restrict enzyme cuts its own recognition sites.
But BsaI recognizes the sequence "GGTCTC"(Figure 1) and cuts downstream of the recognition site as shown in the Figure 2.
And BsaI activity is independent of the sequences of the downstream of the
recognition site.
Restrict enzyme digestion and ligation are completed by just one PCR because once DNA is cut and ligated irreversibly, the recognition site of BsaI disappears.
We create the segments which have complementary ligation sites so that we can introduce plural DNA segments into one plasmid at the same time and arrange them in the way we like . (Figure 4)
Learn more about [http://www.plosone.org/article/info%3Adoi%2F10.1371%2Fjournal.pone.0005553 Golden Gate Assembly].
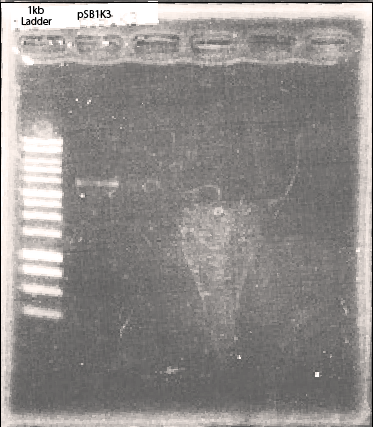
Plasmid backbone [http://partsregistry.org/Part:BBa_K797013 BBa_K797013]
Before we started assembly, we had to make proper gene segments for Golden Gate assembly. The DNA segments we created had four bp for ligation, one base pair spacer, BsaI recognition sites and four base pair at the both ends.
We amplified plasmid backbone(psB1K3).
After amplification, we assembled these DNA segments referring to the protocol.(See the part of Golden Gate Assembly)
After we confirmed this part has the restriction cites and restriction enzyme cutting sites of BsaI. After this, we mixed DpnI with enzyme-treated plasmid and conducted ligation. Other iGEM teams can use this backbone plasmid for their Golden Gate assembly.
Software
We created a software which design primers to create DNA segments for Golden Gate assembly. This software gives you the sequences of primers if you input the sequences of parts and melting temperature.
We created the plasmid backbone [http://partsregistry.org/Part:BBa_K797013 BBa_K797013] and a software to design primers for Golden Gate assembly. They make it easier to use Golden Gate assembly. We want other teams to use this parts and software to use precious time efficiently.
[1]Carola Engler, Romy Kandzia, Sylvestre Marillonnet "A One Pot, One Step, Precision Cloning Method with High Throughput Capability"PLoS ONE 3(11): e3647.
[2]Carola Engler, Ramona Gruetzner, Romy Kandzia, Sylvestre Marillonnet"Golden Gate Shuffling: A One-Pot DNA Shuffling Method Based on Type IIs Restriction Enzymes" PLoS ONE 4(5): e5553. doi:10.1371/journal.pone.0005553 doi:10.1371/journal.pone.0003647
 "
"