Team:Bielefeld-Germany/Results/substrate
From 2012.igem.org
Summary
We tried to degrade our Substrates with the TVEL0 and our self produced Laccases. The HPLC results showed that the hormons are degradeble with our Laccases. Polycyclic Aromatic Hydrocarbons (PAHs) distingrate themself in the Britton buffer but with Laccases there is so little Anthracene that the LC-MS could not detect it. This means that probably the Laccase is able to degradate it. Due to time reasons we could not measure the Analgesics and Lindane which was also one of our Substrates to test but we have not had the opportunity. The spectrofluorophotometer data showed also that Ethinyl estradiol and Estradiol are degraded after Laccase treatment.
Contents |
Spectrofluorophotometer Anaylses
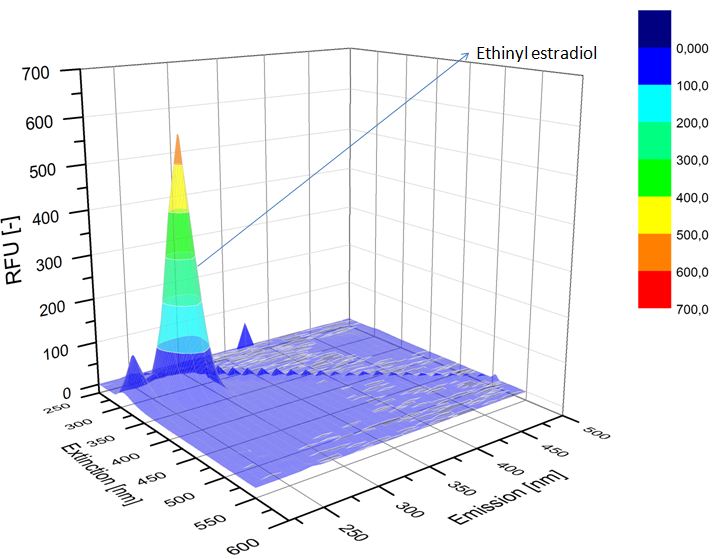
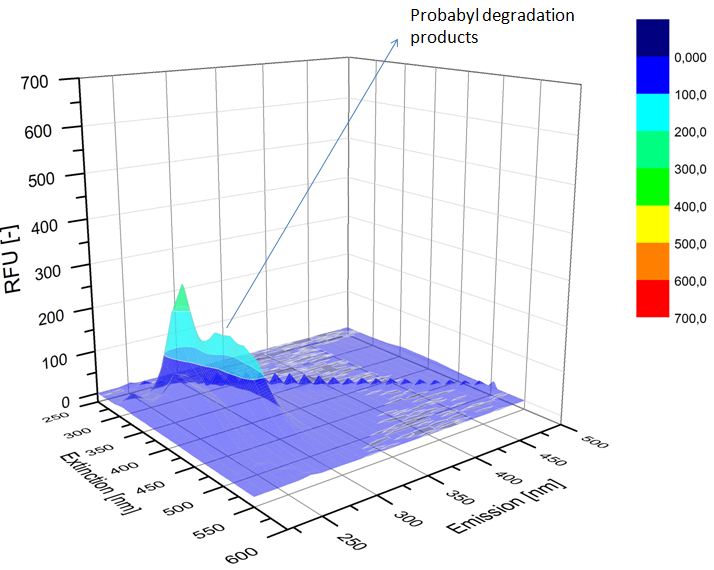
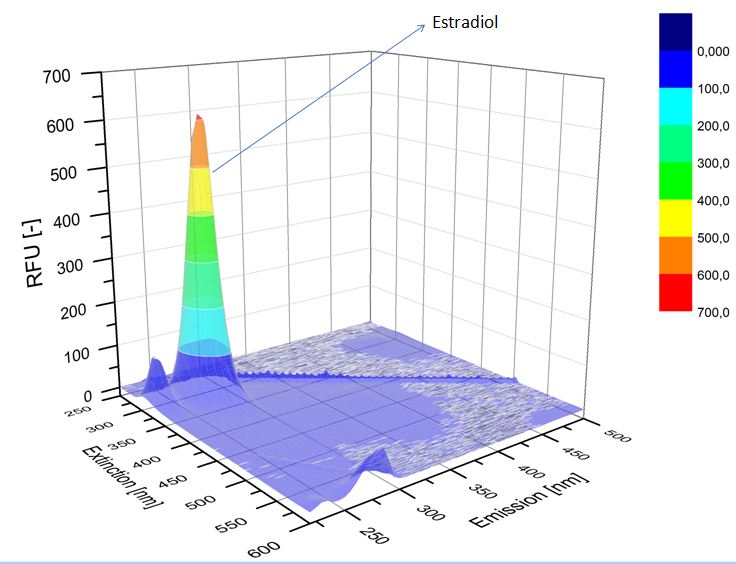
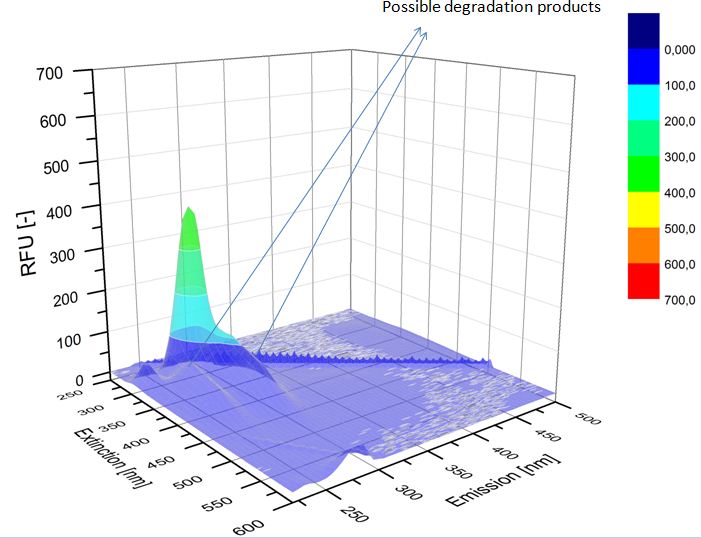
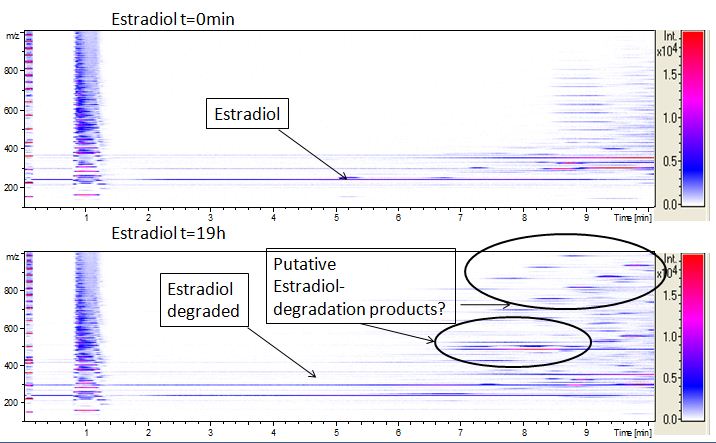
We analyzed our degradation procuts with the spectrofluorophotometer. As you can see it in the figures below the ethinyl estradiol and estradiol are degraded over night. Figure 1 shows the ethiny estradiol without laccase, figure 2 shows that no more ethinyl estradiol is left in the sample after the degradation and new peaks appear, which represent possibly degradation products. In figure 3 you can see the estradiol control without laccases. Like ethinyl estradiol our estradiol peak does not appear in the degradation and new peaks can be seen indicating that those are new degradation products.
Liquid chromatography–mass spectrometry
Dilution series
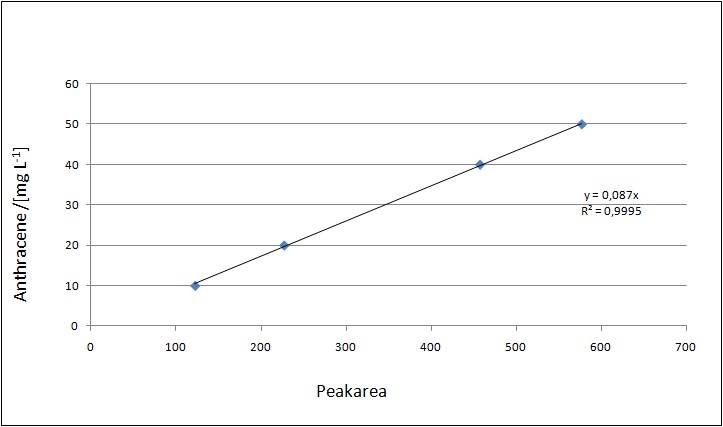
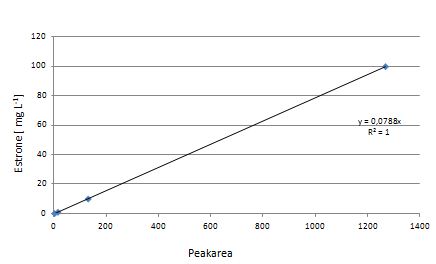
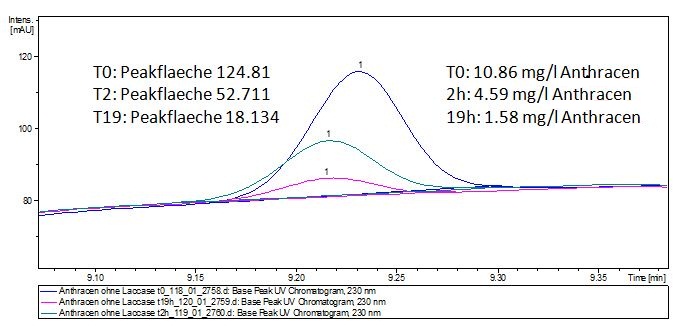
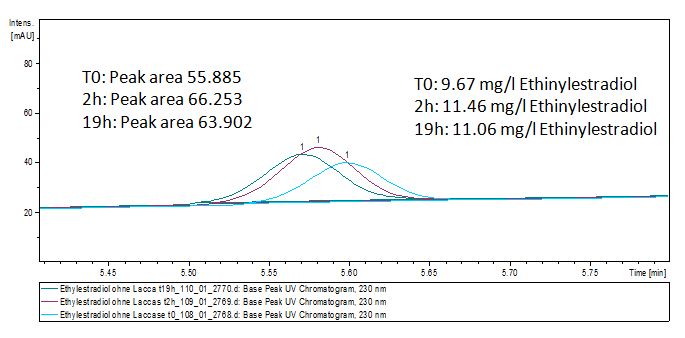
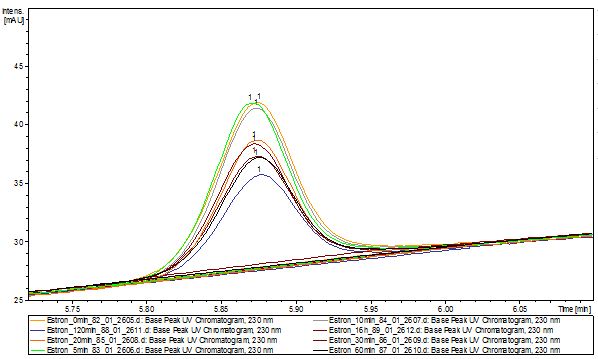
Our substrates are soluble in methanol. We set our standarts into a concentration of 1 mg mL-1. The detection limit for the LC-MS showed that a concentration of 10µg/l for the substrates esteron and estradiol is the highest concentration of detection. The same limit of detection was used for ethinyl estradiol and anthracen for degradation. We only used thoose four substrates. For all LC-MS preperations we used the T. versicolor laccases. The dilution series were prepared in methanol and 50% acetonitril- 50% water.
Degradation results
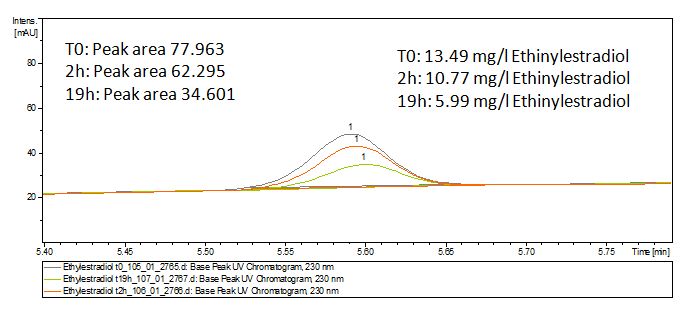
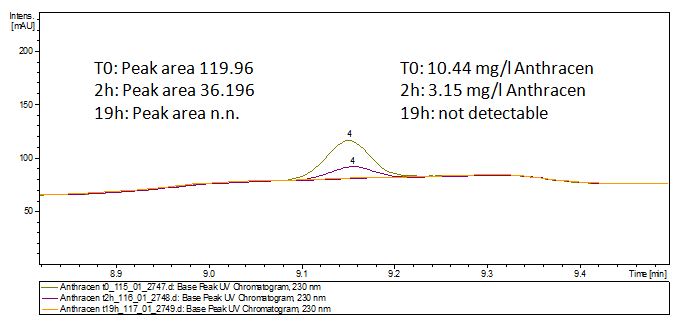
The TVEL0 was able to degradate the synthetic estradiol (fig. 1) and probably also anthracen (fig. 3). Our ethinylestradiol calibration curve showed that it is stable in the media we used (fig. 2). Anthracen distingrates itself in the Britton buffer but with laccases present there is less anthracen measured in the LC-MS indicating our laccase is able to degradate it (fig. 4). Estrone (fig. 5) and estradiol (fig. 6) were degrade as well. On Estrone we could not identify any degradation products. The reason for this could be that the products are not detectable with LC-MS. We could identify peaks in the degradation of Estradiol but were not able to identify them. On the following figures you can see the results of our LC-MS measurments. The controls in different media showed no changes in concentration.
We also tried to measure the degradation with mass-spectrometry. Since quantification via mass-spectrometry is difficult to ionization of the analytes, we quantified our substrats by UV-light. Nevertheless, mass spectrometry enables identification of degradation products. We anaylzed estradiol degradation in detail (fig. 6) resulting in possible chemical compounds generated. Until now we can not say clearly how estradiol degradation functions, but with more time degradation products can maybe identified
High performance liquid chromatography
Dilution series
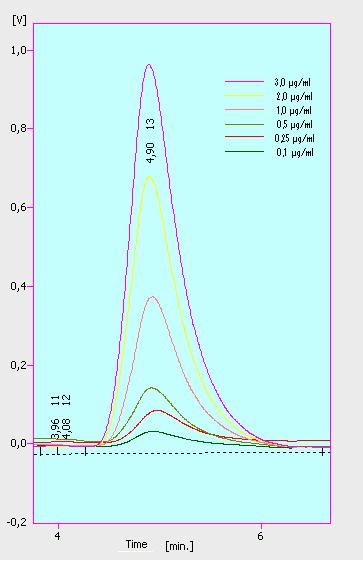
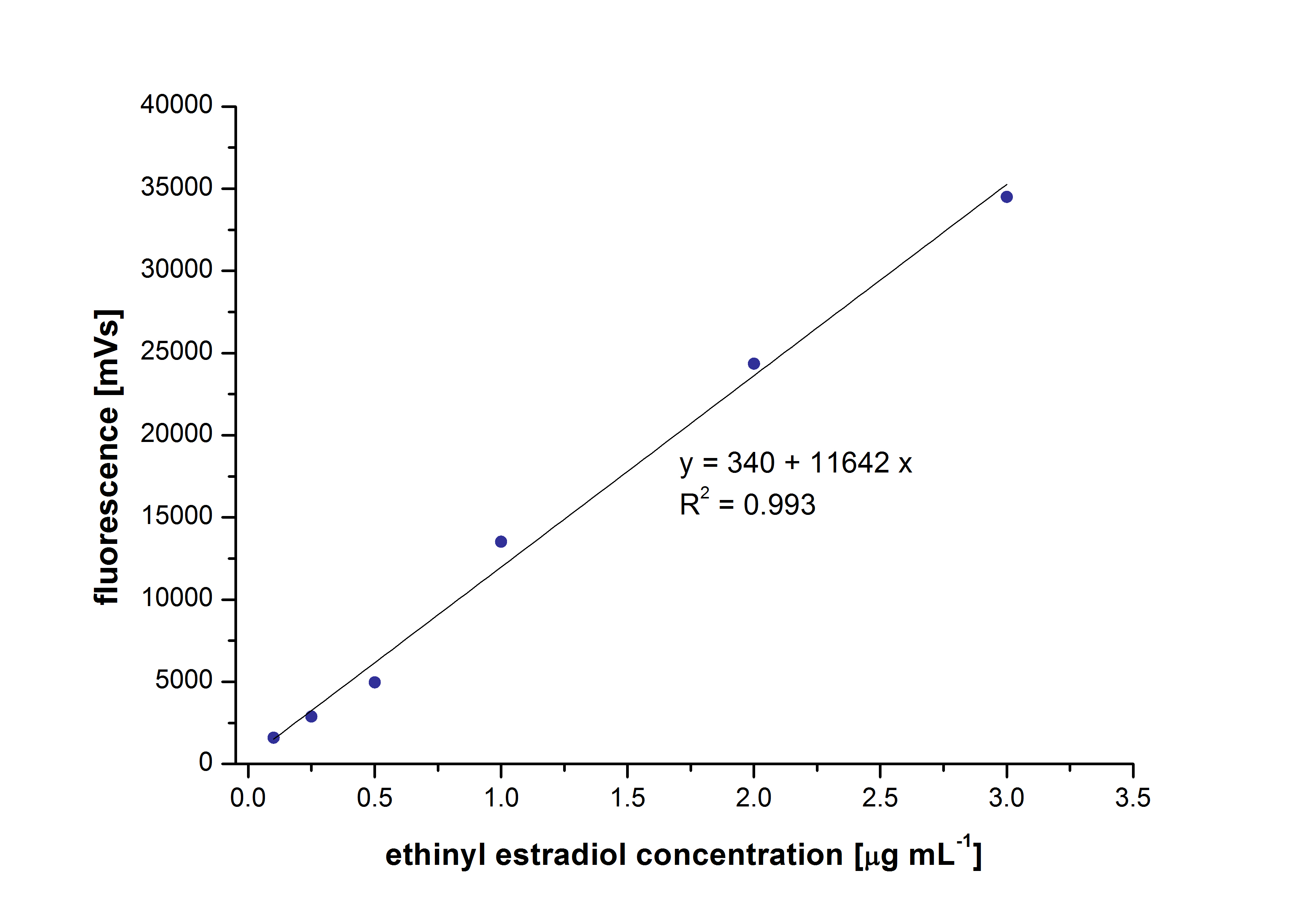
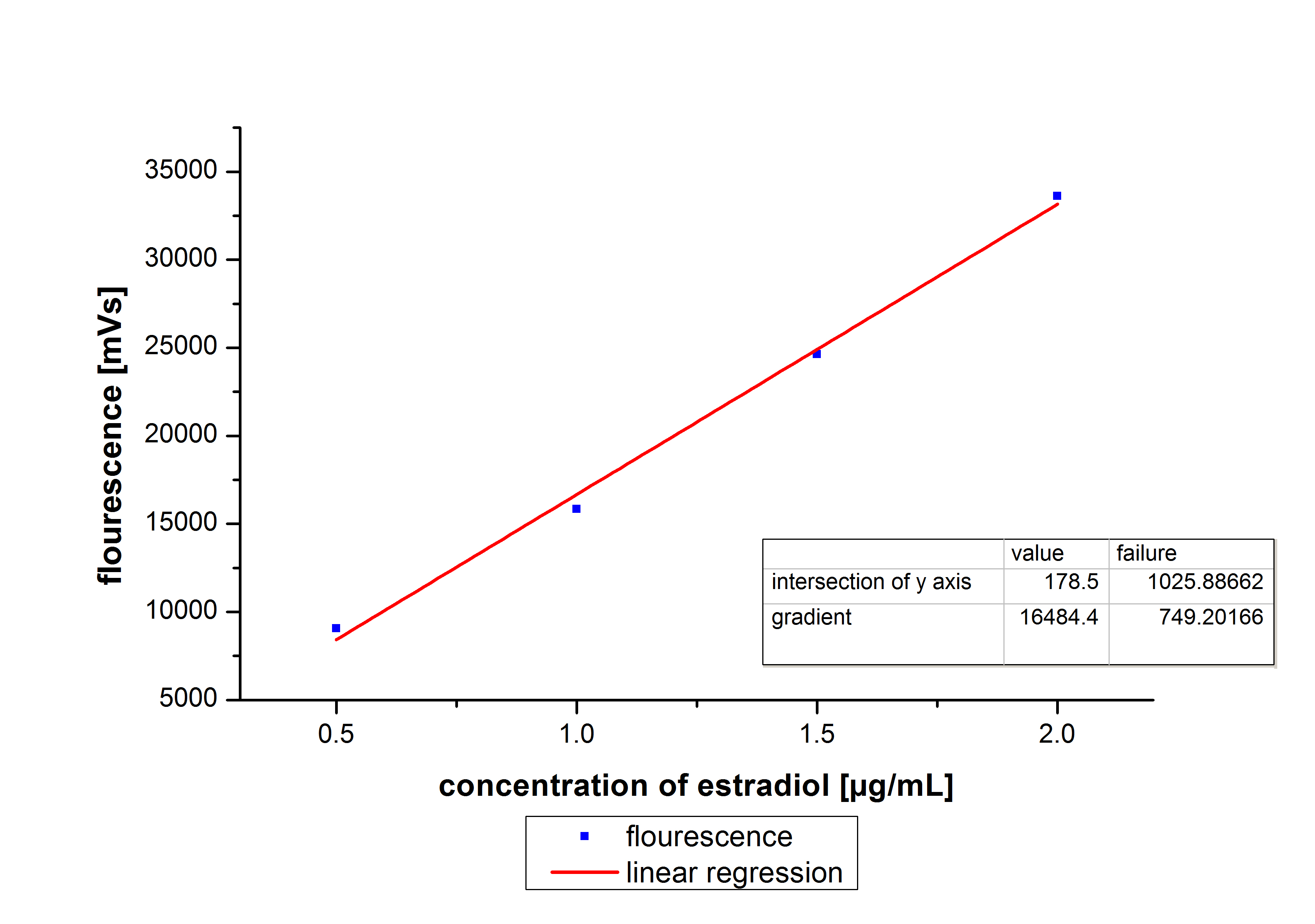
At first we mesured dilution series of all different substrates. While estradiol and ethinyl estradiol where easy to mesure, we didnt succed often with the estrone calibration curves. This was caused by its bad solubility. The retention time for estradiol is 5.8 minutes, for estrone 4.7 minutes and for ethinyl estradiol 5.2 minutes. For all estrogens we could use the same extinction and emmission values: Ex 230, Em 310.
The next substrate class where the analgesics. These three substrates have different optimal extinction and emission values. Additional difficulties occured with naproxene and ibuprofen. Instead of one single peak we found two for each substrate, and none of them correlated with the used concentration. With diclofenac we are still not sure which extinction and emmission values are to use. We found different values and aditionaly we analysed it with a spectrofluorometer but this has also shown no clear peak for diclofenac.
Three of the four PAH´s have the same extinction and emmission values. Simular to the estrogens, the PAH calibration curves are easy to generate. Naphthalene has a retention time of 9.6 minutes and its detection range is also 0.1 to 2.5 µg*mL-1. Acenaphthene with a retention time of 15.1 minutes and phenantrene with a retention time of 17 minutes have maximal detectable concentration of 1.5 µg*mL-1.
Anthracene will be mesured later.
Negative controls
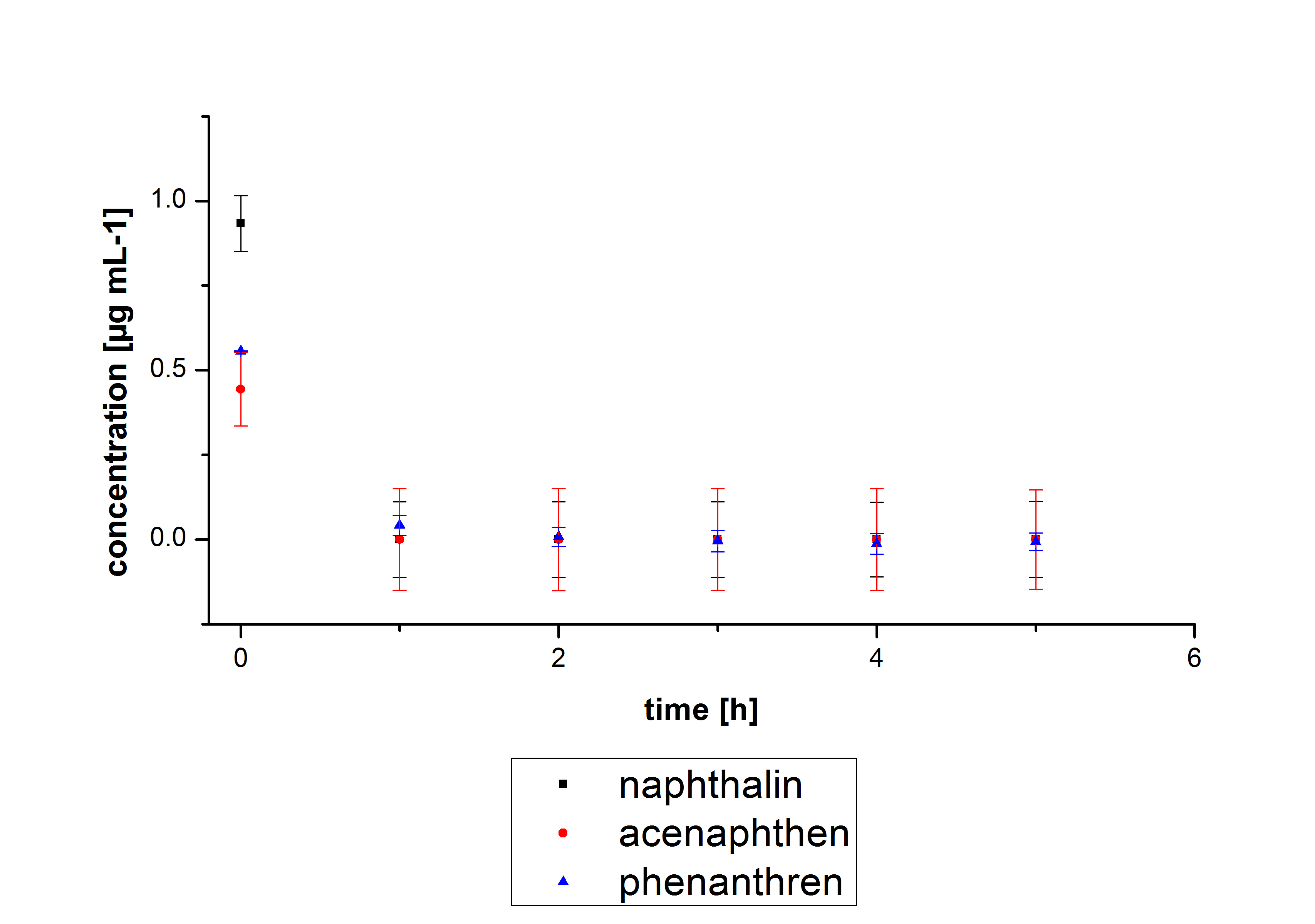
The results of our negative controls of the first Polycyclic aromatic hydrocarbon, PAH, degradations showed, that PAH´s decay without laccase with a high speed. So we take a closer look at the three PAH´s in Britton Robinson (BR)-buffer. The result can be seen in figure 2.1. After one hour most of the used substrates decayed.
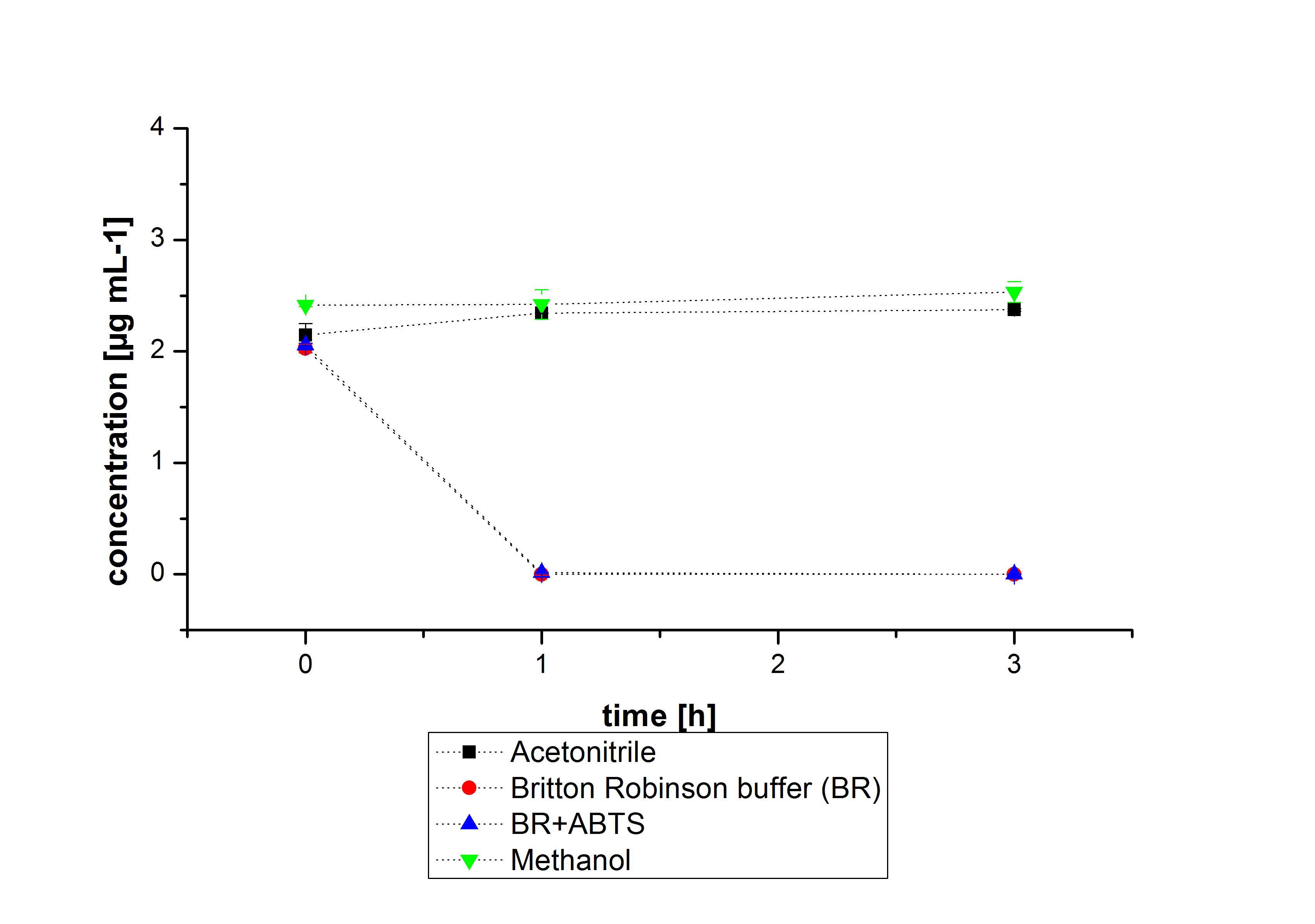
The next step was to check if and which substance cause the decay. In this test we dissolved naphthalene in acetonitrile and in methanol and compared the pure solvents with the influence of the BR-buffer and BR-buffer with ABTS. With pure methanol or acetonitrile naphthalene decays slow in comparison to BR-buffer. In BR-buffer with or without ABTS the decay happens faster and under nearly the same velocity. So BR-buffer seems to be a bad choice to test if our laccases degrade PAH´s.
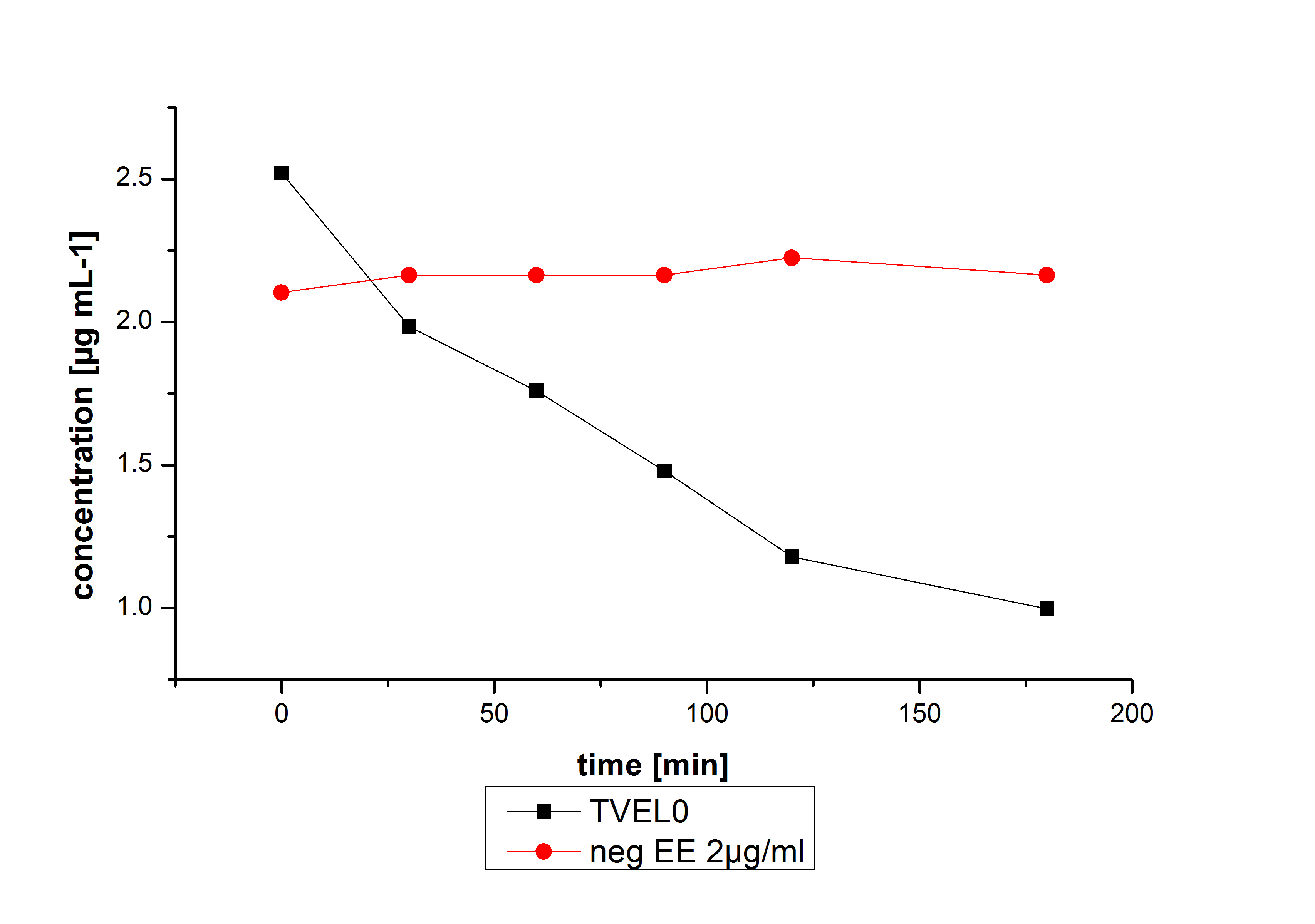
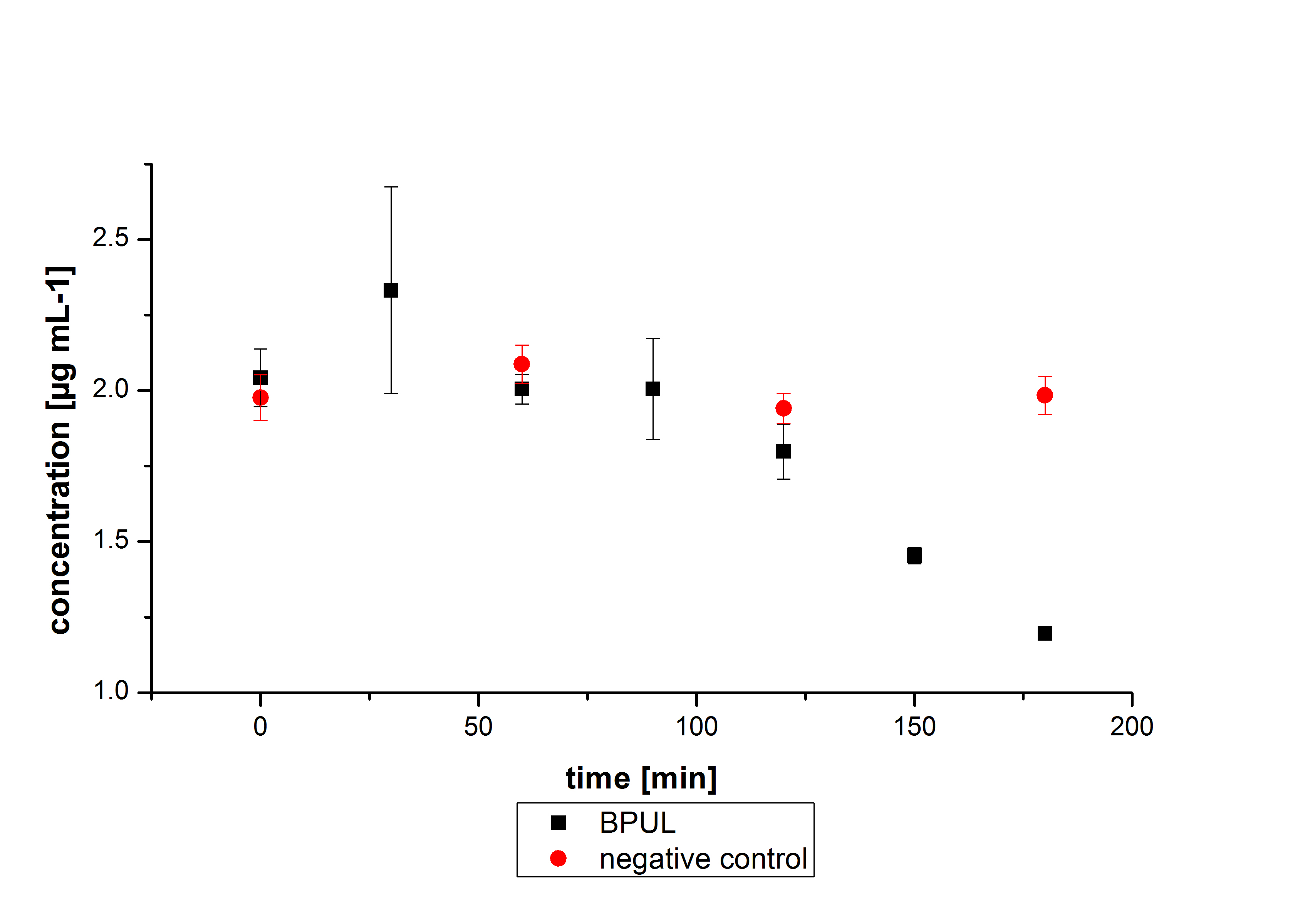
Estradiol and ethinyl estradiol did not decay in BR-buffer, as shown by the negative controls in degradation experiments.
Degradation
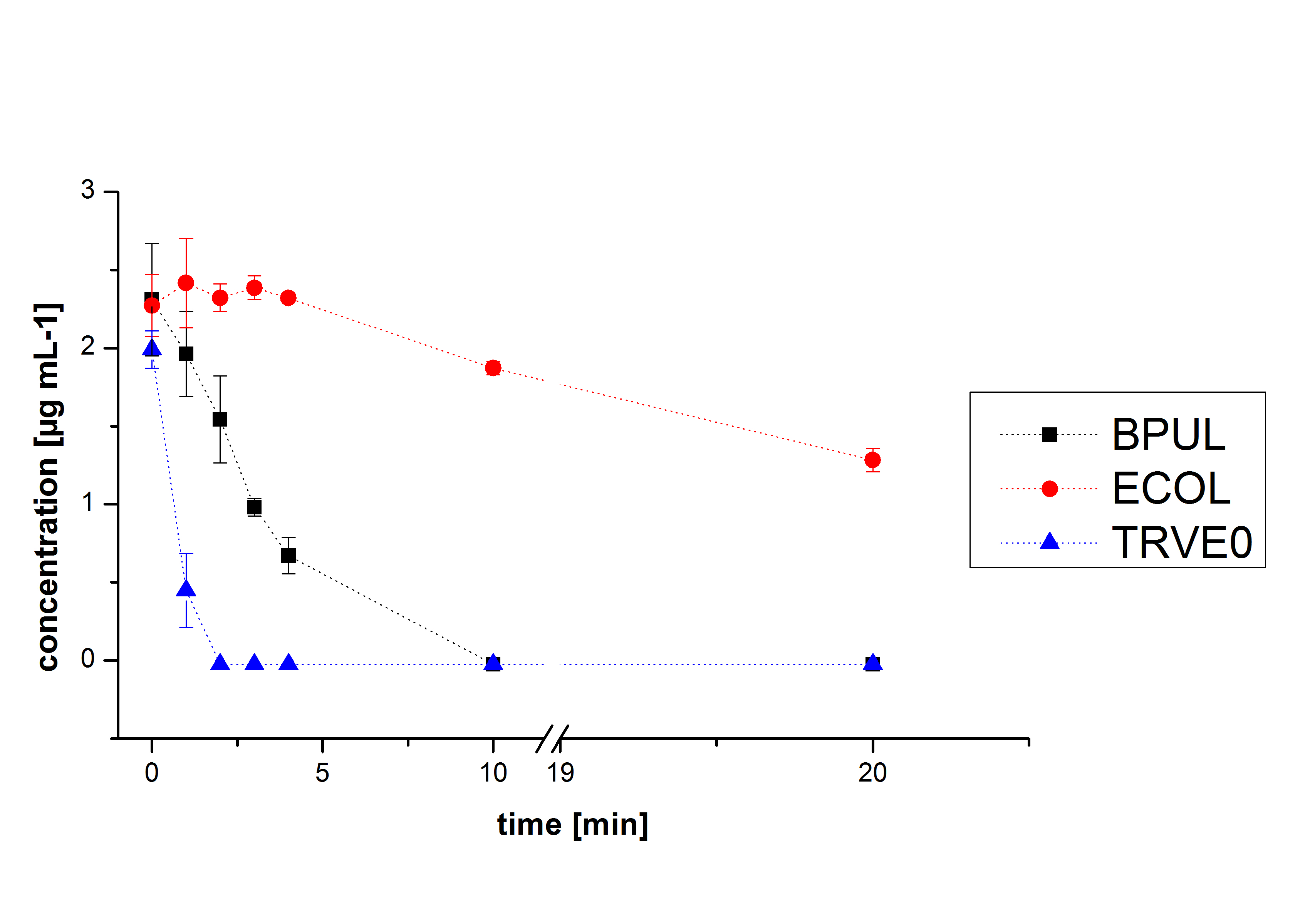
Degradation reactions with ABTS showed the expected results. "Team Activity Test" already showed, that the laccases have the ability to oxidise ABTS. The fact that oxidised ABTS reacts chemicaly with the substrates, explains that all of our active laccases have the ability to degrade ethinly estradiol and other substrates. The potential of the purchased laccase TVEL0 is much higher, shown in figure 3.2. BPUL has not the same potential as the purchased laccase. But BPUL degrades estradiol without the influence of ABTS.
Outlook
Anthracene, lindane and diclofenac are to be detected with the HPLC. Ibuprofen and naproxen need a good calibration curve.
For naphthalene, acenaphthene and phenantrene we have to test different buffers or lower the temperature too keep ist more stable.
We have only tested BPUL and ECOL so far. "Team Activity Test" showed that TTHL is active so we can test this laccase for the different substrates. Also there will be the trametis versicolor laccases TVEL5, TVEL10, TVEL13 and TVEL20.
Additional we want to determine kcat/km for the degradable substances.
On the LC-MS we did not measure our Substrates with our self produced Laccases. If we had the opportunity, the would test our self produced Laccases with the Substrates. Besides we did not measure all Substrates they need to be measured as well to identify degradation products.
| 55px | | | | | | | | | | |
 "
"