Team:Tokyo Tech/Projects/PHAs/index.htm
From 2012.igem.org
Contents |
Achivement
We made a new biobrick part and succeeded synthesizing Polyhydroixyalkanoates(PHAs). In our project, we designed rose silhouette to enhance the balcony scene of “Romeo and Juliet” by the synthesis of PHAs.
What is PHA?
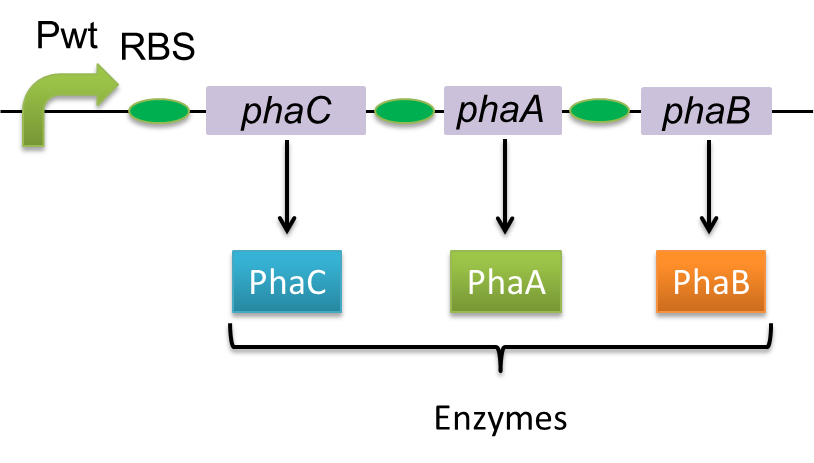
Polyhydroixyalkanoates(PHAs) are biological polyester synthesized by a wide range of bacteria, and can be produced by fermentation from renewable carbon sources such as sugars and vegetable oils. These polyesters are biodegradable thermoplastics and elastomers, which exhibit interesting material properties. Bacteria produce PHAs for the storage of carbon and energy. When the bacteria can’t get enough nutrients from outside, these PHAs will be degraded and used as energy source. This function is quite similar to the one of lipid in human body. PHAs are also a kind of bio plastics, which can be biodegraded a lot faster than fossil-fuel plastics in the environment. Poly-3-hydroxybutyrate, P(3HB) is the most common type of PHAs. P(3HB) is synthesized by the enzymes coded in the gene of PHA synthesis (phaC1-A-B1) from Ralstonia eutropha H16.
Poly-3-hydroxybutyrate, P(3HB) is synthesized by three enzymes.
The A gene encodes for the 393 amino acids protein, 3-ketothiolase (PhaA) The B gene encodes for the 246 amino acids protein, acetoacetyl-CoA reductase (PhaB) The C gene encodes for the 589 amino acids protein, PHA Synthase (PhaC)
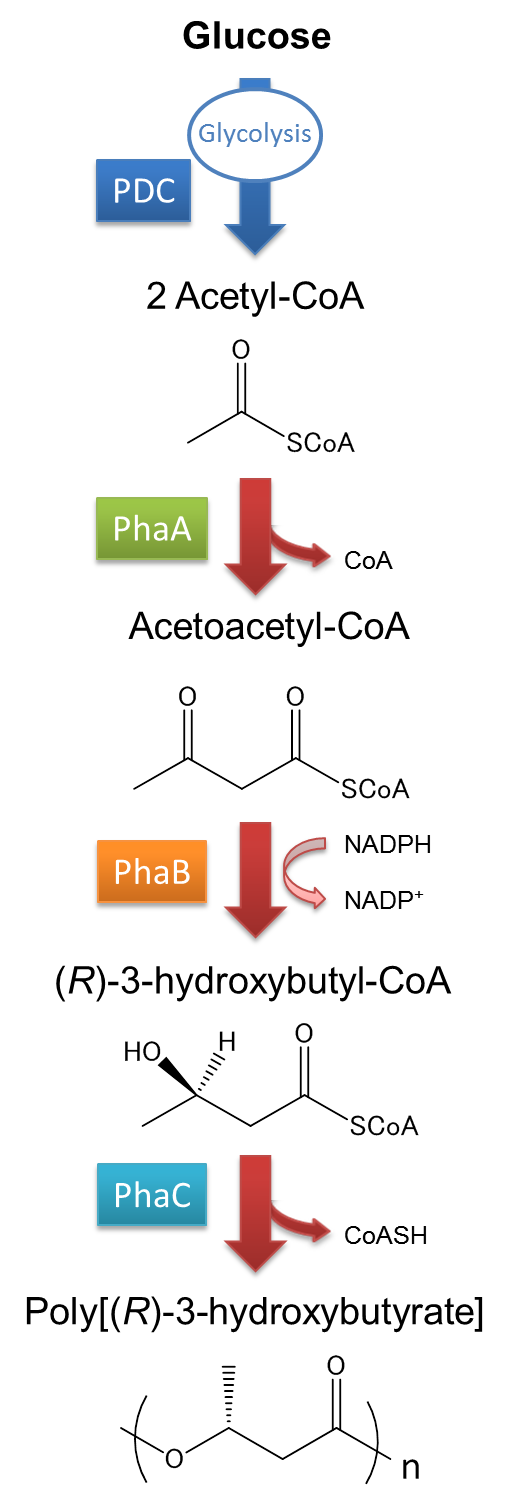
The pathway and regulation of P(3HB) synthesis in Ralstonia eutropha H16 is shown in Fig2. Acetyl CoA is metabolized from glucose by glycolysis and Pyruvate dehydrogenase complex (PDC). At first, two molecules of acetyl-CoA are ligated to one molecule acetoacetyl-CoA by the action of 3-ketothiolase (coded in phaA). Acetoacetyl-CoA is transformed into (R)-3-hydroxybutyl-CoA by NADPH dependent acetoacetyl-CoA reductase(coded in phaB). P(3HB) polymers are then synthesized by the polymerization of (R)-3-hydroxybutyryl-CoA by the action of PHA synthase (PhaC). In most of the cases, PHA synthase determines the characteristic of PHA being synthesized in microorganism.
Other teams
In this study, we constructed a part containing pha-C1-A-B1 in Biobrick format. We introduce some attempts in the past iGEM to show how great our work is in iGEM. There is no Biobrick part which worked as expected though some teams had tried to synthesize PHA in the past.
| iGEM10_Caltech |
| iGEM10_INSA-Lyon |
| iGEM09_Duke |
| iGEM08_UtahState |
| iGEM08_tsinghua |
| iGEM08_Hawaii |
| iGEM08_Virginia |
| Part number | Description | States | experience |
| [http://partsregistry.org/wiki/index.php?title=Part:BBa_K338003 BBa_K338003] | PHA Synthase Composite, Part 1/2 | planning | |
| [http://partsregistry.org/wiki/index.php?title=Part:BBa_K338004 BBa_K338004] | PHA Synthase Composite, Part 1/2 | Available |
They couldn’t prove that their engineered bacteria produced PHB according to the team wiki.
Production of PHAs by E.coli
Construction of pha-C1-A-B1 in Biobrick format
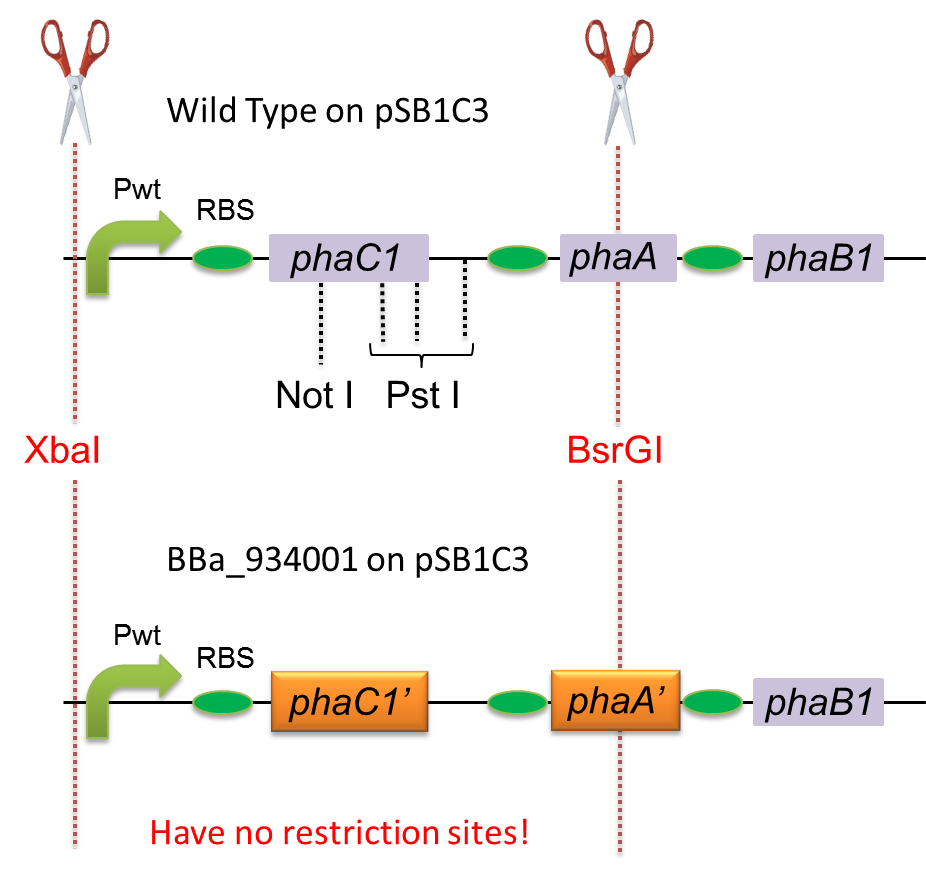
To construct a part that meets Biobrick format, we have modified the pha-C1-A-B1 operon not to contain forbidden restriction enzyme sites. First, we cloned the wild type gene pha-C1-A-B1 from R.eutropha H16 by using PCR and inserted the gene into pSB1C3. However, wild type pha-C1-A-B1 gene sequence contains one NotI and three PstI recognition sites that are not allowed in Biobrick format. To get pha-C1-A-B1 sequence without these recognition sites, we ordered the chemically synthesized DNA from IDT/MBL. In this chemically synthesized DNA, coding is optimized for E.coli. That is to say, we got PHB synthesizing gene in Biobrick format([http://partsregistry.org/wiki/index.php?title=Part:BBa_K934001 BBa_K934001]).
PHB production by E.coli&Confirmation of PHB
To synthesize PHB by E.coli, we transformed E.coli JM109 with the constructed phaC1AB1 parts on PSB1C1(BBa_K934001). JM109 is used to synthesize PHB, because it tends to have a high density accumulation of PHB.As a negative control, we transformed E.coli JM109 with plasI-gfp on PSB1C1 .
(ⅰ) We observed the accumulation of PHB in the E.coli colonies on Nile red positive medium under UV .Nile red has been widely used to stain colonies and distinguish between PHA-accumulating and non-accumulating colonies. Nile red produces a strong orange fluorescence (emission maximum,598nm) with an excitation wavelength of 543nm (maximum) upon binding to PHA-granules in cells of R.eutropha (Degelau et al.1995) H16. Nile red in the agar medium doesn’t affect the growth of the cells, and the occurrence of PHAs in the colonies can be directly monitored. This method is quite sensitive and results in fluorescent colonies of PHA-positive strains. So we cultured the transformant on LB agar medium plates with 0.5μg/ml Nile Red and 2% glucose at 37℃ for 30 hours, then we transferred the plates to 4℃ room. After 115 hours, colonies with PHB would be stained Red by Nile red when observed under UV. This showed that the transformant had stored PHB.FIG1 is the photograph of E.coli(with phaC1AB1 gene) colonies on Nile Red positive medium taken under UV. The fluorescent area in the figure showed the accumulated PHBs stained by Nile red in cells. This indicated that part BBa_K934001 functioned correctly.FIG2 is the photograph of negative control cells. In this figure we observed that there was no remarkable fluorescent area.
(2)Nile Blue
Text
Perspective
Text
 "
"