Team:Cornell/Notebook/Wetlab
From 2012.igem.org
| Home | Team | Project | Parts | Modeling | Notebook | Protocols | Safety | Attributions |
|---|
|
June
June 12th-16th
June 12th, Tuesday
June 13th, Wednesday
- Set up PCR for four fragments of the nah operon as well as the pBMT-1 backbone, using primers designed to mutate cut-sites concurrently with Gibson Assembly. Also set up a PCR for the entire nah operon. Due to length of the fragments, a longer extension time was chosen.
- If Gibson Assembly fails, but we are able to PCR the entire 10kb nah operon, an alternate method of mutation using three sequential site-directed mutageneses will be pursued.
- Work done by: Caleb, Claire, Dylan, Spencer, Steven, and Swati
June 14th, Thursday
- PCR only amplified nah1, nah3, and nah4 - longer fragments (nah2, the full nah operon, and pBMT-1) were not identified on the PCR product gel. Set up another PCR with shorter extension time.
June 15th, Friday
- PCR of four out of five products visible on gel. Set up PCR of pBMT-1, final fragment required for Gibson Assembly of the nah operon.
June 16th, Saturday
June 17th-23rd
June 17th, Sunday
- Successful PCR of pBMT-1 gel purified.
June 18th, Monday
June 19th, Tuesday
- Ran Gibson Assembly of nah operon fragments into pBMT-1 backbone and transformed into DH5a electrocompotent E. coli cells.
- Set up PCR to amplify the Gibson Assembly products.
- Work done by: Dylan and Swati
June 20th, Wednesday
- Set up a digestion of the PCR of Gibson Assembly products and ran the digested products on a gel to see if Gibson worked.
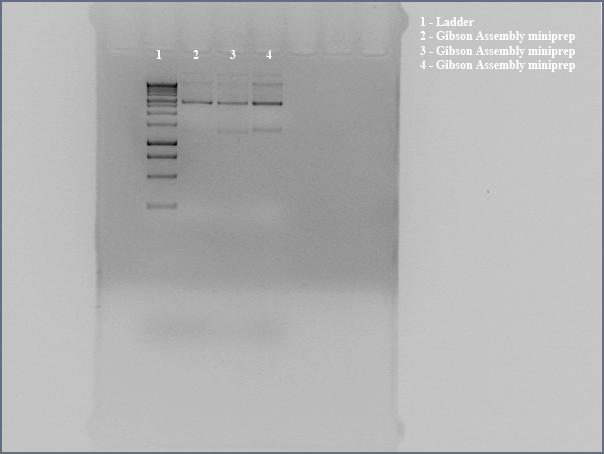
- Three colonies on plates of DH5a transformed with Gibson Assembly product. Made liquid cultures to miniprep and sequence.
- Work done by: Dylan and Swati
June 22nd, Friday
- Miniprepped directly transformed Gibson Assembly product for sequencing using the the Gibson nah1F and nah4R primers (each w/ 20 bp overhangs).
- Ran undigested miniprep with gel electrophoresis, looking for large bands corresponding to supercoiled DNA. The gel shows distinct bands for all three lanes. We interpreted this to mean that we got product. Submitted for sequencing
June 23rd, Saturday
June 24th-30th
June 24th, Sunday
June 25th, Monday
June 26th, Tuesday
June 27th, Wednesday
- Ran digest of gibson-assembled nah operon with gel electrophoresis
June 28th, Thursday
- Gel purified arsR construct
June 29th, Friday
- Vent PCR at 11:00 (DPW)
- Amplifying both previous Phusion PCR band and original p21 template
- Dylan's magic triple anneal method (55/60/63)
- Gel purified PCR product from Phusion template (~1:20 pm) (DPW)
- Quantified product at 22.4 ng/uL
- Set up digestion of p21 PCR product with EcoRI-HF and AscI (~9:00 pm).
- 22 ng/uL --> 45.5 uL sample for 1 ug digest
- Buffer 4
- Ran digestion on gel. (~11:00 pm)
- Sliced out relevant band on gel, stored overnight at -20.
- Miniprepped overnight cultures of PL14-PL20 (~1:00 pm, STC)
- Using C1015 rotor, 6666 x g, the Corning culture tubes only fit in the centrifuge with the lids off
- Made LB, 3x 60 mL in 100 mL bottles (~ 3:00pm, SS)
- Made LB Agar, 4 x 250 mL LB Agar in 500 mL bottles (~3:00, CS)
- Autoclaved LB, LB Agar, and milliQ (~3:30 pm, SS)
- Made LB plates with Kan (~6:30 pm, CMR)
- CUGEM movie outing at 8:00 pm.
- Phusion PCR at 10:00 pm (DPW)
- Dylan's magic triple anneal method (57/65/70, 35 cycles total)
- Amplifying nah operon from Gibson 1
- Appending BioBrick cutsites for ligation into pSB3C5
June 30th, Saturday
- Took PCR out of thermal cycler at 9:00 am (DPW)
- Set up gel using NEB 100bp and 2-log ladders (10:00am)
- Gel extracted PCR product, quantified at ~10ng/uL
- Set up new Phusion PCR using Gibson 1 as template
- Dylan's magic three-anneal method (57.6/65/72)
- Extension time of 3 min.
- Continued gel extraction of p21 PCR digest from previous day (SS)
- Set up ligation of p21 PCR digest extraction and dephosphorylated pBBRBB+mtrB (11:11am, SS)
- Desalted ligation using Millipore membrane paper
- Transformed 2 electrocompetent DH5alpha stocks at 5:30 pm and 5:50 pm, respectively.
- Observed time constants for electroporation of 4.38 ms and 4.24 ms, respectively
- Let cells recover for 1 hour, plated on LB + Kan.
- Set up two ligations of pSB3C5 into PNNL electrocompetent Shewanella strain JG700 (6:30 pm, Sp.C and St.C)
- First transformation performed at usual PNNL voltage of 0.75 kV (time constant of 9.30 ms)
- Second transformation performed at Myers and Myers specification of 0.55 kV (time constant of 9.34 ms).
July
July 1st-7th
July 1st, Sunday
At 9:50, Dylan and Caleb set up 2 gels for electrophoresis. Caleb's was 1% agarose in BIO-RAD Mini-Sub Cell system for continuation of ladder test using SYBR Green, containing NEB 100 bp ladder, NEB 2-log ladder, Promega 1 kb ladder and run at 100V. Dylan's was 1% agarose in Owl box using ethidium bromide, containing the nah operon PCR product from previous night, and run at 55 V.
Our plates of DH5alpha transformed with p21 ligated into pBBRBB with mtrB grew only one colony, possibly contamination. Dylan ran a colony PCR and got a smear, suggesting that the PCR of p21 or the ligation did not work.
Because we learned that our SYBR Green was causing ladder to run strangely, Dylan decided to redo a Vent PCR to amplify the salicylate reporter region out of p21.
Also, a liquid culture of JG700 was prepared, as well as replating of p21, p22, JG700, JG1220, JG1537, JG1219. (See: strain list)
July 2nd, Monday
Today, Dylan and Caleb ran a gel of a Vent PCR of p21 (the PCR product being the salicylate reporter) at 55 V. Additionally, Caleb decided to run a control gel at 100 V to determine whether higher voltage was a factor in our previous ladder problems (in addition to the SYBR Green stock). While we determined that the higher voltage did not cause our ladder issues, we did not see any bands from the p21 PCR.
Dylan prepared electrocompetent cells using the Myers and Myers protocol. Modified protocol using 2 5mL cultures @ 4000g for 10 min. Washed with 2 mL sorbitol, resuspended in 100 uL sorbitol. First electroporation ts = 2.32 ms, second ts = 2.02 ms. Added 1 uL of plasmid (575.5 ng) to each cuvette. First used .60 V, then 0.55 V, both with R = 400 ohms.
Mark and Danielle started liquid cultures of S1, S9, S10, S11, S15, S16, S18, S27 (See: strain list)
July 3rd, Tuesday
Caleb miniprepped S16 (p15), S22 (p21), S9 (p8), S10 (p9), S11 (p10), S18 (p17), and S15 (p14). (See: strain list) Instead of a single EB elution, Caleb did two elutions, 30 ul each. The double 30 ul elution turned out to be effective at recovering a usable amount of DNA in the second elution, so we're including it on our miniprep protocol for future minipreps.
The rerun of our Gibson sequencing failed again, so we tried a more roundabout method of determining whether our 3 Gibson transformants have complete nah operons or not. We digested them each with BamHI, expecting specific fragment lengths on a gel electrophoresis.
When Dylan and Mark were attempting to determine what volume of ethidium bromide should be used in the gel, they came to the conclusion that better images may result from staining the gel in dilute ethidium bromide after running the gel, rather than including the stain in the gel. This experimental first staining used 200 mL of water conaining 20 uL of 10 mg/mL ethidium bromide, with gentle agitation for 1 hour. This stained the bands well, but also provided a large amount of background and took a very long time.
We only saw one band on each, all of them at about 3.6kb.
Because no transformations from our previous competent freezer stocks were successful, Dylan decided to make a starter culture of Shewanella strain JG 700 in preparation for making new competent stocks using the PNNL Protocol. Swati and Tina used this starter culture to complete the preparation.
July 4th, Wednesday
In the morning, Dylan, Swati, and Danielle prepared p8, p9, p10, p14, and p15 for sequencing (from the minipreps that Caleb preformed the previous day).
The team took the rest of the day off and had a barbeque at Buttermilk Falls. There was an excess of food, spontaneous song, and fireflies.
July 5th, Thursday
Upon arriving to the lab, Dylan dropped off the sequencing tubes we'd set up on the morning of the 4th for analysis. He also decided to do a Colony PCR of the potential transformant with the Salicylate reporter plasmid. However, when setting up this reaction, Dylan realized that we'd used the wrong sequencing primers (we'd used the standard VF2 BioBrick primer instead of a custom primer for the pBBRBB backbone). Consequently, Mark and Dylan resubmitted p14 and p15 for sequencing while the colony PCR was ongoing. After visualizing the PCR products using DNA Gel Electrophoresis, Dylan concluded that the colony we'd screened did not have our plasmid of interest, and was the result of contamination.
Similarly, Dylan noticed small, evenly spaced colonies on all of the JG700 transformant plates from the Myers and Myers procedure we'd undertaken on the 2nd of July. Because none of the colonies looked red (as they should have if they were replicating pSB3C5), Dylan concluded that the chloramphenicol on the plates had degraded, and the colonies were resultant from untransformed cells.
Because the colony PCR of the potential salicylate reporter didn't yield good results, Dylan and Youjin set up another Phusion PCR to amplify the salicylate-sensing region from p21. Simultaneously, Mark made SOB media for recovery after future transformations, as we'd just received the ingredients for the broth.
Because we needed to submit for sequencing twice (because we used the wrong sequencing primers), Swati et al. set up liquid cultures of p21, p8, p9, p10, p17, and p14 in order to have more DNA in the freezer. We like having DNA in the freezer. (See: strain list)
July 6th, Friday
Caleb miniprepped p21, p8, p9, p10, p17 from the liquid cultures set up the previous night. (See: strain list) We couldn't miniprep p14, since – after checking notes from the previous night – we learned that the culture was made with the wrong antibiotic (chloramphenicol instead of kanamycin).
Some sequencing results came back, however the Gibson results are still confusing.
Also, a gel was run for the p21 PCR product.
July 7th, Saturday
July 8th-14th
The focus of this week was to transfer our arsenic sensing plasmids to Shewanella strain JG700 via congugation with E.coli strain WM3064. After struggling to electroporate control plasmids, we consulted with Dr. Gralnick who reccomended proceeding with conjugation and kindly provided E.coli strain WM3064. This strain is auxotrophic for 2,6-Diaminopimelic Acid (DAP), which makes the strain useful for selecting against non-Shewanella conjugants.
July 8th, Sunday
July 9th, Monday
Dylan performed a Phusion PCR with a single annealing temperature of 59 degrees Celsius using primers 27 and 28 for the final time. In the future, new primers will be used. The purpose of this PCR was to amplify p21 (See: strain list). The PCR product was analyzed using agarose gel electrophoresis to determine if the desired 1.127 kb fragment is present. To transform our plasmids into Shewanella with conjugation, we had asked Dr. Jeff Gralnick for WM3064 E. coli for which plates containing 2,6-Diaminopimelic Acid (DAP) are required. In preparation to receive this strain, the plates with DAP and DAP/kanamycin were prepared.
July 10th, Tuesday
Caleb miniprepped strains containing plasmids p8, p9, p10, p15, and p16 (See: strain list) Also, our new freezer arrived. We cleaned and welcomed our new friend.The WM3064 came in and we plated from the agar stab. Dylan set up two 18 mL/hr continuous flow reactors using 10x diluted LB and innoculated one of the reactors with JG700 and one with wild type Shewanella oneidensis MR-1.
July 11th, Wednesday
Dylan started a WM3064 subculture in the morning before meeting Swati, Shweta, Caleb, and Danielle at the Boyce Thompson Institute for Plant Research to give a presentation both on our project and on the broader applications of synthetic biology to the energy industry to a group of high school teachers from New York state. After the presentation, Dylan and Danielle prepared plates containing DAP/chloramphenicol and DAP/ampicillin to grow transformed WM3064 on, while Mark continued preparing electrocompetent stocks of WM3064 with the subculture that Dylan had started.
We transformed things later that day.
July 12th, Thursday
Shweta prepared a glycerol stock of WM3064 for future use, and Dylan plated some remaining transformed WM3064 hoping to get more colonies of the successfully transformed cells.
Dylan and Mark submitted p8, p9, and p10 for sequencing using two different primers for each plasmid.
The previous night's p21 PCR seemed to have failed, even with the new primers. Based on the gel run by Claire, then analyzed by Dylan and Caleb.
July 13th, Friday
Today began with Dylan and Caleb setting up conjugations between our transformed donor E. coli and S. oneidensis.
The sequencing results for p8, p9 and p10 all came back as failed, so it was concluded that the Gibson assembly failed. There were five locations on the nah operon at which sequencing could begin, of which we considered all but the fourth. Sequencing at the first location resulted in a sequence that corresponded to the fifth sequencing location, with some sequence before it and all of the expected sequence after it. The fifth sequencing location produced the expected sequence. Then the middle two regions which we considered all resulted in failed sequencing. This has led us to the conclusion that segments that are required in the operon are missing, and thus the assembly failed. Dylan and Caleb began attempting to look into the possibility of this being a cause, and some potential solutions.
In discussing why p21 PCR seems to continue failing, the idea of having p21 sequenced arose. Dylan prepared the sample and submitted it for sequencing. Meanwhile, Mark set up yet another Phusion PCR of p21, this time with a higher annealing temperature of 61 degrees Celsius and a decreased extension time of 18 seconds. The goal was to prevent the mispriming which seemed to have occurred in the previous day's attempt.
Later that day, Dylan set up yet another PCR with a decreased annealing temperature of 55 degrees Celsius in order to empirically narrow-in on the optimal annealing temperature for the amplification of the salicylate sensing region from p21.
July 14th, Saturday
Swati ran a gel of 5uL of the p21 PCR that Mark set up yesterday. After staining, the DNA ladder was well visualized but there were no bands in the p21 lane, suggesting that the PCR did not work. In a fit of rage, Swati yelled at Maneesh for going to get a drink of water - yet another innocent victim of failed PCR. Then, after a full team meeting, we all came together for a dumpling party. Noodles, dumplings, and gnocchi of all kinds were eaten by the voracious team. In the spirit of true, unwavering scientific inquiry, we conducted a novel experiment whilst feasting on these delectable treats. The result: fried Dorito dumplings are delicious.
July 15th-21st
July 15th, Sunday
Dylan noticed that we had colonies on the plates re-streaked from the original plates of conjugated S. oneidensis. He started overnight cultures of S20 (See: strain list) in kanamycin so that we can sequence and make glycerol stocks. He also started overnight cultures of w.t. S. oneidensis to innoculate into new reactors in Riley Robb, which will be used as positive controls.
July 16th, Monday
The overnight cultures of S. oneidensis need more time to grow - overnight cultures of these leisurely bacteria should be started at noon the previous day, instead of in the evening as with speedy E. coli. Dylan ran another Phusion PCR of the entire nah operon, since we got a lot of mispriming the first time we ran it. The thought was that we had run the previous PCR at the optimal temperature for Vent but used Phusion, so Dylan set up the new PCR correctly, with a single annealing temperature of 66 degrees Celsius and a lengthened final extension time of 15 minutes to account for the size of the nah operon (~10kb). Caleb ran a gel of the PCR and visualized a single band ~9.5kb. PCR of the nah operon out of p20, P. putida (See: strain list) was successful! Tina and Swati gel extracted and quantified, extracting two samples at 38.8 ng/uL and 27.2 ng/uL.
Spencer miniprepped p14, our arsenic reporter part (arsR + mtrB w/ BamHI cutsite), from S20 (See: strain list) for sequencing, recording yields of 44.3ng/uL & 35.8ng/uL (colony 1) and 50.6ng/uL & 53.5ng/uL (colony 2) from quantification.
Dylan and Tina set up transformations of p15 and p16 (See: strain list), the arsenic reporter part without a BamHI cutsite, so that we can conjugate S. oneidensis in the next few days. They also set up a transformation of the miniprepped BBa_J01003 with the oriT mobility gene. Our new method of inserting plasmids into S. oneidensis, using conjugation rather than electroporation, requires that all our plasmids have the mobility gene. However, the plasmid we were going to use for the nah operon (pSB3C5) has no mobility gene. Possible approaches to take are to clone oriT from iGEM kit plates into pSB3C5, or to PCR the mobility gene out of one of our own plasmids and clone that into pSB3C5. We will try to get oriT from kit plates first.
July 17th, Tuesday
Shweta set up a Phusion PCR of p21 (See: strain list) to try again to extract the salicylate-sensitive promoter. Sequencing of p21 showed that there was a stem loop sitting upstream of the ENX biobrick cutsites, which wasn't expected and is most likely why our primers are not effective. Before redesigning primers, we are going to try PCR with the standard iGEM forward sequencing primer and the same reverse primer that we designed for p21.
July 18th, Wednesday
Dylan set up p15k, p16k conjugation plates from overnight cultures of transformed WM3064 and JG700. Incubated 8 hours, streaked for single colonies on kan plates with E.coli and Shewanella controls. Caleb miniprepped p24a (BioBrick part with oriT) from overnight culture. Desalted overnight ligation mix and transformed DH5a (nah operon in pSB1C3).
Restreaked... p26a p30a p27a p29a
Set up reactor in Riley Robb.
Spencer made overnight cultures of p25a, p28a, p31c.
July 19th, Thursday
Only observed 2 possible colonies from transformants from yesterday's ligation (nah operon in pSB1C3). Restreaked from one of these colonies along with a DH5a control.
We got single colonies on the JG700+p15 and JG700+p16 plates, and no growth on control plates. Tentative conclusion is that conjugation was successful. Made reference plates of picked colonies, and grew up liquid cultures from same colonies for sequencing.
Dylan and Caleb miniprepped p25a, p28a, p31c from overnight cultures, then made glycerol stocks of S25, S28, and S31.
Inoculated reactor in Riley Robb with S20, our engineered arsenic reporter strain.
Digested p21 PCR product along with miniprepped p14 with EcoRI and AscI. Purified p21 PCR digestion with Omega Bio-Tek E.Z.N.A. MicroElute DNA Clean-Up Kit. Dephosphorylated p14 digestion with Antarctic Phosphatase, and ran entire mixture on a gel. (Picture of gel). Purified 6kb band with Qiagen gel extraction kit.
Overnight cultures of a bunch of stuff.
July 20th, Friday
Shweta and Tina miniprepped p26a, p27a, p29a, p30a (Anderson series constitutive promoters), and p14k from overnight cultures.
Dylan set up 30 minute room temperature ligation to construct salicylate reporter with digested p21 PCR product and p14 (isolated yesterday, quantified today). Not confident that ligation will work because of incredibly low yields from digestion cleanup and gel extractions.
Because Shewanella grows more slowly than E.coli, Claire did a second miniprep of p15k and p16k from JG700 in order to submit plasmid for sequencing to confirm that conjugation was successful.
In anticipation of the failure of Dylan's ligation, Swati set up 3 Phusion PCR reactions in parallel, each with the same template (p21), repeating the parameters that had proven successful (no mispriming) previously.
Dylan set up overnight cultures of... nah operon in pSB1C3(?) S18 (DH5a + pSB3C5, p17c) WM3064+p14
July 21st, Saturday
July 22nd-28th
July 22nd, Sunday
July 23rd, Monday
Dylan quantified the gel extractions, quite successfully with a newly invented protocol. Then he dephosphorylated p14, and submitted some DNA for sequencing. At one point, the Magical Graduated Cylinder of Elmira (500 mL) fell from the sky and shattered in the sink. Some voodoo was clearly in the air. It appears that the ligation products from the day before were either not created or not successfully transformed, as the plates contained no colonies.
Caleb and Tina prepared more kanamycin and chloramphenicol plates while Mark desalted Dylan's ligation product. Then Dylan, Mark, and Tina transformed DH5&alpha with the ligation product, p33k, p14k, and p31c (See: strain list).
July 24th, Tuesday
July 25th, Wednesday
Mark set up two ligations of a p20 PCR product and p17c, one at standard concentrations and one at very high concentrations. (See: strain list) These were desalted and transformed by Mark and Dylan, along with p33k. Meanwhile, Tina and Chie set up double digestions of p24a, p20 PCR product, and single digestions of p25a, p27a, and p29a.
Dylan ran two gels of the double and single digestions. The single digestions of the Anderson series promoters showed single bands, which slightly faster than the supercoiled DNA control. This may be because the supercoiled DNA was nicked. The gel of the oriT double digestion showed more long bands than expected, but had a band ~400bp which was interpreted to be the insert of interest.
July 26th, Thursday
Caleb gel purified the digestion products of p24a, p20 PCR< p25a, p27a, and p29a.
July 27th, Friday
July 28th, Saturday
July 29th-31st
July 29th, Sunday
July 30th, Monday
July 31st, Tuesday
August
August 1st-4th
August 1st, Wednesday
August 2nd, Thursday
August 3rd, Friday
August 4th, Saturday
August 5th-11th
August 5th, Sunday
August 6th, Monday
August 7th, Tuesday
August 8th, Wednesday
August 9th, Thursday
August 10th, Friday
August 11th, Saturday
August 12th-18th
August 12th, Sunday
August 13th, Monday
August 14th, Tuesday
August 15th, Wednesday
August 16th, Thursday
August 17th, Friday
August 18th, Saturday
August 19th-25th
August 19th, Sunday
August 20th, Monday
August 21st, Tuesday
August 22nd, Wednesday
August 23rd, Thursday
August 24th, Friday
August 25th, Saturday
August 26th-31st
August 26th, Sunday
August 27th, Monday
August 28th, Tuesday
August 29th, Wednesday
August 30th, Thursday
===August 31st, Friday===
 "
"