Team:Yale/Project
From 2012.igem.org
| Home | Team | Official Team Profile | Project | Parts Submitted to the Registry | Modeling | Notebook | Safety | Attributions |
|---|
Contents |
David's write-up
Confirmation of the universality of the pBAV1K-T5 vector
In order to determine whether the pBAV1K-T5 vector (Bryksin and Matsumura, 2010) would serve as an appropriate universal backbone for our cassette, we did a cross-species transformation experiment with the unaltered pBAV1K-T5-gfp plasmid. For E. coli, we used XL1-Blue cells that were made chemically competent, and for the other two species we used naturally competent cells. The success of transformation was gauged by selection on LB-Kan plates, with a concentration of 20 μg/mL for B. subtilis, and 50 μg/mL for Acinetobacter baylyi and E. coli.
What we found was that the XL1-Blue and ADP1 WT cells were consistently transformed (i.e., growth when expected, selective colonies), while the four B. subtilis strains (168, PY79, PERM739, and 1A833) showed no indications of successful transformation despite several different attempts. While it is possible that pBAV1K-T5 simply does not replicate in high enough copy numbers in B. subtilis, it seems more likely that the problem lie somewhere in the transformation protocols. We initially used a two-step transformation protocol from Harwood’s Molecular Biological Methods for Bacillus, and then tried electroporation, then a one-step transformation method also from Harwood’s protocols. The results were consistently poor, with very little reproducibility for any positive results obtained. Upon correspondence with the authors, we learned that they too had trouble yielding consistent results for B. subtilis.
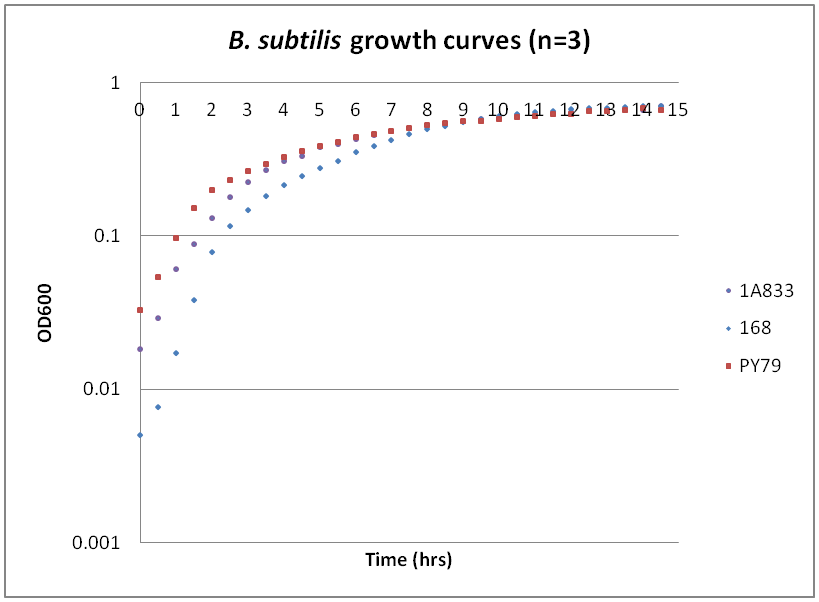
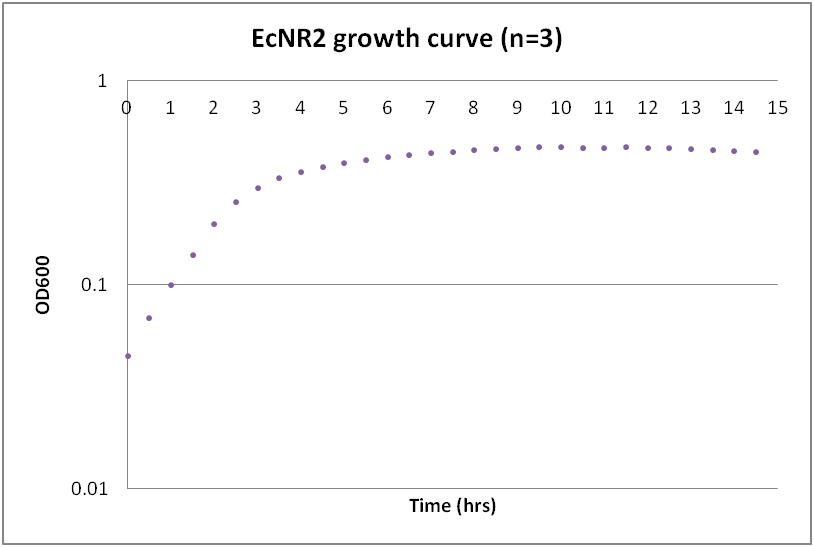
Growth curves for Ac, Bs, and Ec
While troubleshooting the unsuccessful transformation of pBAV1K-T5 in B. subtilis, we thought that it would be beneficial to obtain growth curves for the strains that we were working with. Unlike WT A. baylyi, which is constitutively competent at the stationary phase (12-16 hrs), B. subtilis requires more specific conditions for natural competence, namely starvation and stationary phase growth. Since each of the protocols that we looked at called for slightly different OD600 values – each set of which were largely for the WT strain (168) – we thought that characterizing the growth of all the B. subtilis strains would provide more insight into the optimal conditions for transformation. The growth curves here were generated by growing starter cultures (n=3) of each of the strains in a 24-well plate that was kept incubating in a plate reader at 34⁰C with shaking for 14.5 hours. The OD600 values were recorded automatically every 5 minutes, but the plots show the points at half-hour increments for clarity. What we found was that each of the different strains of B. subtilis showed notable differences in the mid- to late-exponential phase of growth, a fact that can be used in further efforts to transform our cassette-containing vector into B. subtilis via natural competence.
 "
"