Team:Peking/Project/Luminesensor
From 2012.igem.org
Introduction
To date, scientists equipped with optogenetic tools have been able to manipulate cellular behavior to achieve some truly fascinating goals such as tuning the expression of genes through the intensity of light, making bacteria capable of detecting the edge of a light illuminated pattern, illuminating specific positions on the cell to trigger pseudopod formation or cell polarization, or even recruiting fluorescent proteins to cell membrane to print patterns on the cell.
However, as we have described in project overview, several defects still hinder the future application of existing optogenetic tools, namely low sensitivity, narrow dynamic range, and the requirement of exogenous chromophores. To offer a satisfactory solution to all of these problems, the 2012 Peking iGEM team set out to develop a novel optogenetic tool that would possess high sensitivity, wide dynamic range, and independency of exogenous chromophores all at the same time.
To achieve this ambitious goal, we followed a general design principle of attaching a physiological functional domain to a photosensor domain to achieve lignt-inducible physiological function, while choosing and engineering each domain carefully and rationally.
For the photosensor domain, we chose the VVD protein, the smallest photosensor protein known, which forms a rapidly exchanging dimer when excited by blue light. For the physiological domain, we chose the LexA protein, a bacterial transcription inhibitor, whose DNA binding domain rigorously requires the help of the dimerization domain for its DNA binding activity. We fused these two separate domains together to form a fusion protein, which we termed the “Luminesensor”. The VVD domain of the Luminesensor will form a dimer after sensing blue light and help the DNA binding domain of LexA to dimerize, thus facilitating its DNA binding activity. So our Luminesensor will function as a blue-light-activated transcription repressor that will inhibit the expression of LexA responsive genes when exposed to blue light. (See Design).
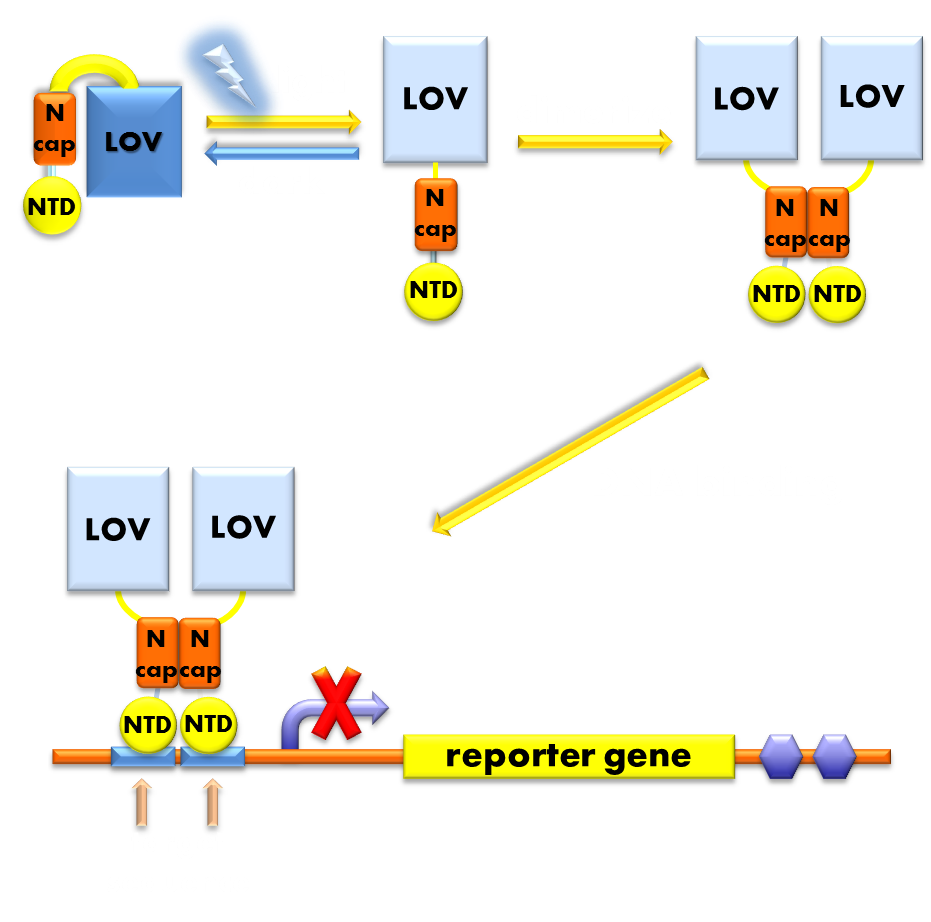
Figure 1. Illustration of the function mechanism of our luminesensor. When exposed to blue light, the N-terminal cap of VVD domain will undock and cause the VVD domain to dimerize. The dimerization of the VVD protein will help the dimerization of N-terminal domains of LexA protien fused to the N-terminal of the VVD domain. When helped to dimerize, the NTD of the LexA protein will recognize and bind to the SOS box in the promoter of our reporter gene and inhibit transcription initiation.
After building our Luminesensor, we moved on to characterizing its sensitivity and dynamic range. To our astonishment and satisfaction, we found that this brand new Luminesensor was able to sense light as dim as bio-luminescence and respond in a switch-like manor with a dynamic range of over 150 fold. We also found that the Luminesensor capable of working properly in E.coli without any supply of exogenous chromophores. These thrilling facts declared our victory, for our design was able to achieve high sensitivity, wide dynamic range, and independence of exogenous chromophores all at the same time, fulfilling our original criteria. (See Characterization).
However, this is not the end of the tale. We realized that the Luminesensor will be interfered by the endogenous bacteria SOS system, since any promoter that is controlled by the Luminesensor will be inevitably inhibited by the endogenous LexA protein. Therefore, Luminesensor will only be able to function in ΔLexA bacteria strains, which will greatly impede our Luminesensor’s future application. Fortunately, we found that a LexA408 variant recognizes a symmetrically altered target sequence different from the sequence that wild-type LexA would recognize. Thus, we postulated that by reshuffling our LexA DNA binding domain to a 408 mutant form, we would be able to ensure that our Luminesensor’s orthogonality to the host genetic context. (See Characterization)
We chose two LexA responsive promoters psulA and precA, and attached GFP reporter downstream of the 408 mutant form of these two promoters. We then tested them in both wild-type and Δ-LexA E.coli strains. Our result proved that our LexA408-VVD Luminesensor is perfectly orthogonal to the endogenous SOS system, while our 'wild type' LexA-VVD Luminesensor is severely interfered by the endogenous LexA protein. (See Characterization)
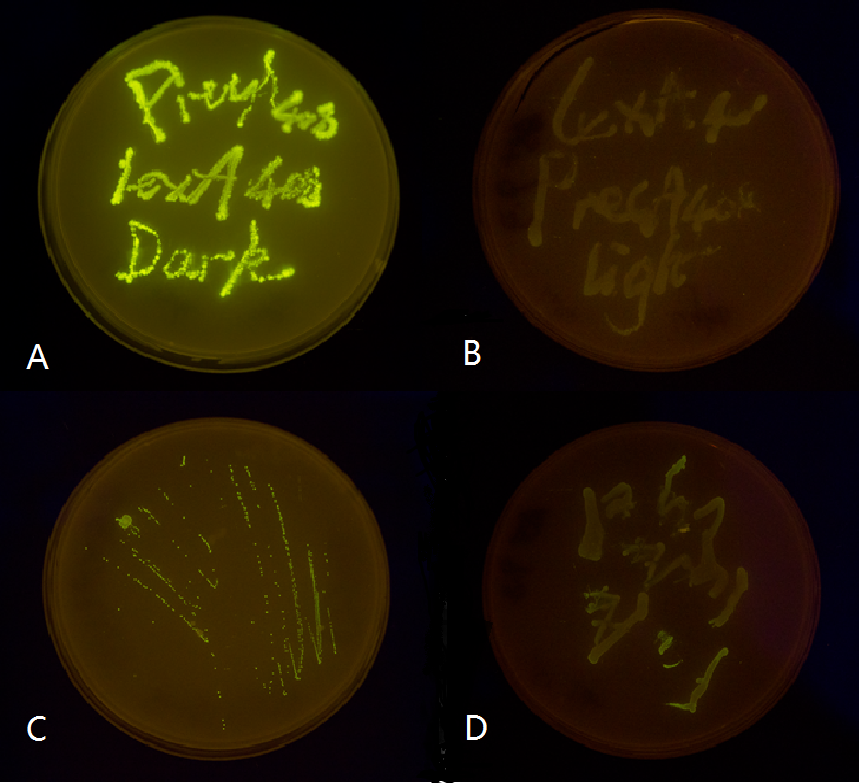
Figure 2. Plate streaked with wild type E.coli transformed with mutated luminesensor and GFP gene fused to the downstream of precA408 and psulA408 and cultivated in the dark or under light.(A: luminesensor + precA408-GFP, cultivated in the dark. B: luminesensor + precA408-GFP, cultivated under light. C: luminesensor + psulA408-GFP, cultivated in the dark. D: luminesensor + psulA408-GFP, cultivated under light.) Just as we predicted, the reporter gene is expressed in the dark and supressed by our luminesensor under light. This clearly proved that mutated Luminesensor works orthogonally to host genetic context while retaining light-controllability.
Though our Lumiesensor outshines many previously designed optogenetic modules, there is still space for improvements, such as its slow decaying half-life.
So we constructed an in-silico reaction network model for our Luminesensor’s light-induced transcription repression function process, and analyzed sensitive parameters. Based on modeling results, we searched through literature for VVD mutations that would substantially improve those sensitive parameters. We then incorporated those mutations into our Luminesensor and discovered that its dynamic performance was further enhanced.(See Design)
 "
"