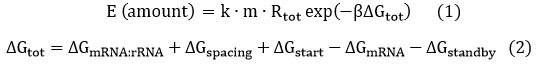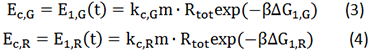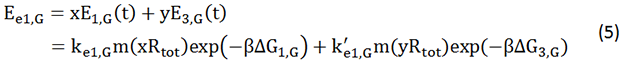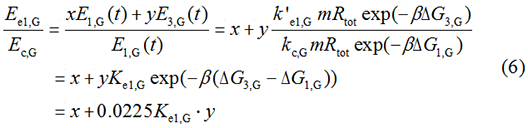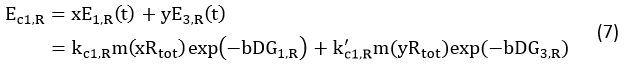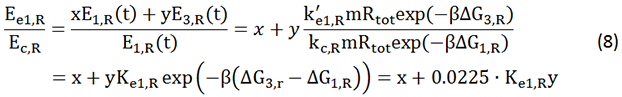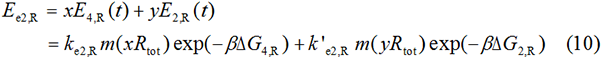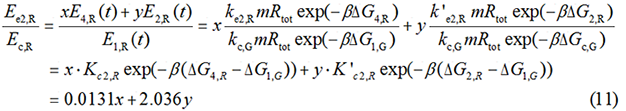Team:Tianjin/Modeling/Calculation
From 2012.igem.org

Contents |
Calculation and Derivation of the Protein Expression Amount Model in Three States
Overview
Three problems came up while we start calculating: what sequences to input, which method to use and how cogent the result will be. As for sequence, we input the both the SD and the protein coding sequence.
Our goal is to calculate the total ΔG of each reaction and then predict the amount of protein expressed. There are several softwares dealing with DNA or RNA base-pairing progress, such as NUPACK, RBS-Calculator, and Vienna RNA etc. After comparison, we decide to use RBS-Calculator.
Due to the complication of translation progress and our lack of insight in this issue, the results of our modeling can’t be very precise. But at least it should have the precision of order of magnitude.
Input of Our Calculation
RBS sequence
- RFP, normal RBS
ATTTCACACATACTAGAGAAAGAGGAGAAATACTAGATGGCTTCCTCCGAAGACGTTATCAAAGAGTT
CATGCGTT
- RFP, orthogonal RBS
ATTTCACACATGTTCCGTACTAGATGGCTTCCTCCGAAGACGTTATCAAAGAGTTCATGCGTT
- GFP, normal RBS
TACTAGAGAAAGAGGAGAAATACTAGATGCGTAAAGGAGAAGAACTTTTCACTGGAGTTGTCCCAAT
TCTTGTT
16S rRNA sequence
- normal 16S: ACCTCCTTA
- orthogonal 16S: ACGGAACTA
Formula Derivation
Basic Idea
For model design, please refer to Design part. Data of the curve Er-time is obtained from Ec=K∙Er experiments. The function of our model is to work out the proportion factor 'K'.
Basic Assumption
- The expression of RFP and GFP are independent.
- The expression of the two proteins are determined by the percentage of normal and orthogonal ribosomes rather than the number of the two ribosomes.
- The growth curve of bacteria do not change significantly after the transferred into orthogonal protein expression system.
Formula Derivation
We start from Formula 1 and 2. In Formula 1, m stands for the number of mRNA transcript, Rtot is the total number of ribosomes, β is the apparent Boltzmann constant, ∆Gtot is the total change of Gibbs free energy, k is proportion factor.
Because the GFP and RFP coding sequence are on the same mRNA transcript, the values of m of GFP and RFP are always same. In different state and time, the total number of ribosomes varies. We assume that at the same time Rtot of different state remain same. Calculation of DGtot is the main job of this model. The proportion factor 'k' represents all unknown factors. Here we assume that as for the same protein in deferent state 'k' varies little.
The Calculation
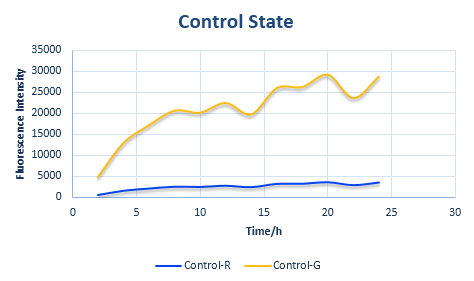
Control State (c)
The two formulas above serve as denominators in following deduction. We get series of disjointed data of Function 3 and 4 through experiments. The amount of GFP and RFP can't be measured directly so we measured the fluorescence intensity of each protein. And because all the formula in this model are based on a singular cell, we must consider the influence caused by the number and growing condition of bacteria.
Experimental state 1 (E1)
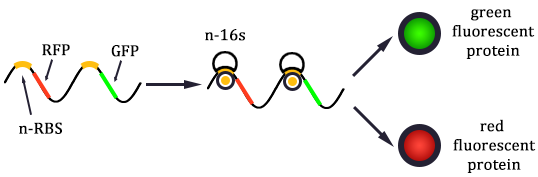
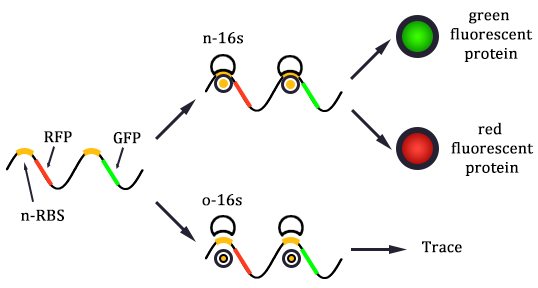
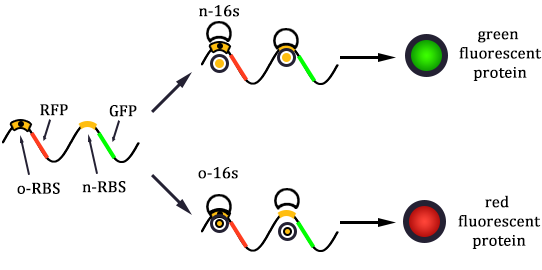
The expression of GFP is composed of two part, the expression of n-RBS::n-16S and of n-RBS::o-16S.
There is no difference for the expression of GFP in Experimental state ONE from the control state, except for the distribution of ribosomes. For this reason, we presume that kc1,G and kr,G are nearly equal and thus Kc1,G equal to 1. The same thought is also shown in following derivation.
We noticed that the proportion factors in equation 6 and 8 are equal, which is not a coincident. This is because ΔG3,G-ΔG1,G≈ΔG3,R-ΔG1,R. The difference of ΔG3,G and ΔG1,G.
Experimental state 2 (E2)
Note : Strictly speaking, the factor K should better be obtained from experimental data rather than assumed to be 1 for such simplification could lead to much deviation from real value.
The Calculation of ΔGtot
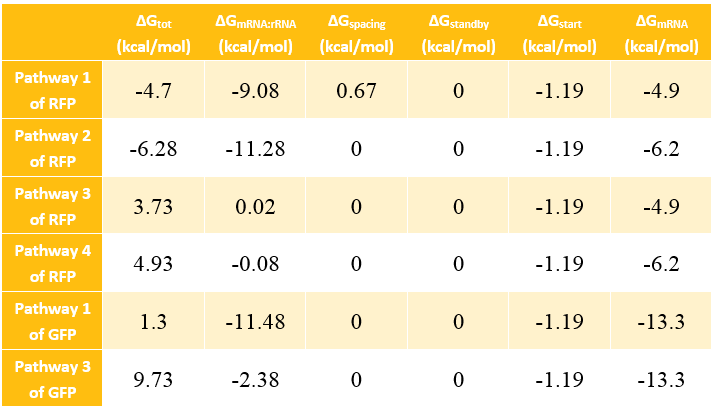
In most cases, the difference of delta G among the four pathways are mainly reflected by the ΔGmRNA-rRNA. There are two ways to calculate the ΔGmRNA-rRNA:
- With the help of RBS calculator;
- Use the method in literature Computational design of orthogonal ribosomes.
There are some differences between the input and output of the two method.
1st method:
- input:
- standby + RBS + Spacing + Start Codon + Protein-Coding,
- 16S 3' last nine bases;
- output: ΔGmRNA:rRNA, ΔGstart, ΔGspacing, ΔGstandby, ΔGmRNA;
- Software: RBS-Calculator;
- Strength: taking more factors into our consideration and are more accessible to real condition;
- Weakness: not sure of the input sequence.
2nd method:
- input:
- ASD sequence,
- SD sequence (each are 6 bases long);
- Output: ΔG under different conditions;
- Software: RNA-Cofold in Vienna RNA web servers;
- Strength: only need to input the SD and ASD sequence which is very easy;
- Weakness: not as accurate as the first method.
Similarity of the two method:The core program of RBS-Calculator is based on Vienna RNA.
There are also two points that should be focused on during our modeling:
- the specification of input;
- the analysis of errors if there are any in the output.
As for analysis of errors, the most frequent errors are the Long-Range Paring which occurred when the head and the tile of the mRNA sequence complement with each other within the sequence itself. In such case, the ΔGmRNA result is not accurate. We usually use RNA-Fold to calculate the accurate ΔGmRNA to avoid such error.
Calculation Results of ΔG
Prediction of Protein Expression Amount
We should, first of all, measure the relative protein expression amount (fluorescence intensity) and to obtain the parameters in the model through regression. After the obtaining the model parameters, we can predict the amount of protein expressed and to compare them with that of wet lab results.
With the calculation result from the previous relative expression formula, together with the wet lab result of fluoresce intensity of RFP which is mentioned in the notebook, we can have the exact value of percentage of normal ribosome x.
After the calculation, we can obtain that x=0.9737, y=0.0263.
When determine the relative expression level, the GFP intensity should be the same for experiment state one and state two. However, there are some divergences between the GFP intensity of a single cell between the two states. We rationally assume the divergence in the total intensity is result from the number of bacteria (in other words, the OD value leads to the different GFP intensity). So the intensity of RFP for a single cell should also be adjusted.
We can put the value of x and y into the formula of the relative protein expression ratio. What is more, we also measured the Florence intensity curve of the control state. With the obtained value of the ratio we have predicted the curve of the experimental state one and the experimental state two. The result figure of our prediction process is shown in the following four figures.
Model Extension
In the previous protein expression model, we just suppose the existence of the orthogonal expression system do not have any significant impact on our system. However, to make our system more accurate, we also need to take the factor of estimation.
Analytical hierarchy process (AHP)
What is AHP?
The analytic hierarchy process (AHP) is a structured technique for organizing and analyz-ing complex decisions. Based on mathematics and psychology, it was developed by Thomas L. Saaty in the 1970s and has been extensively studied and refined since then.
It has particular application in group decision making, and is used around the world in a wide variety of decision situations, in fields such as government, business, industry, healthcare, and education.
Rather than prescribing a "correct" decision, the AHP helps decision makers find one that best suits their goal and their understanding of the problem. It provides a comprehensive and ra-tional framework for structuring a decision problem, for representing and quantifying its ele-ments, for relating those elements to overall goals, and for evaluating alternative solutions.
Is there any application in real life?
While it can be used by individuals working on straightforward decisions, the Analytic Hierarchy Process (AHP) is most useful where teams of people are working on complex problems, especially those with high stakes, involving human perceptions and judgments, whose reso-lutions have long-term repercussions. It has unique advantages when important elements of the decision are difficult to quantify or compare, or where communication among team members is impeded by their different specializations, terminologies, or perspectives. Decision situations to which the AHP can be applied include:
- Choice - The selection of one alternative from a given set of alternatives, usually where there are multiple decision criteria involved.
- Ranking - Putting a set of alternatives in order from most to least desirable
- Prioritization - Determining the relative merit of members of a set of alternatives, as opposed to selecting a single one or merely ranking them
- Resource allocation - Apportioning resources among a set of alternatives
- Benchmarking - Comparing the processes in one's own organization with those of other best-of-breed organizations
- Quality management - Dealing with the multidimensional aspects of quality and quality improvement
- Conflict resolution - Settling disputes between parties with apparently incompatible goals or positions
The AHP procedure
Calculation result through AHP method
IGEM Project evaluation model
Determination of the Optimum propaganda method
Universal Risk evaluation model of Environmental problem
Reference
- Saaty, Thomas L.; Peniwati, Kirti (2008). Group Decision Making: Drawing out and Rec-onciling Differences. Pittsburgh, Pennsylvania: RWS Publica-tions. ISBN 978-1-888603-08-8.
- Saaty, Thomas L. (2008-06). Relative Measurement and its Generalization in Decision Making: Why Pairwise Comparisons are Central in Mathematics for the Measurement of Intangible Factors - The Analytic Hierarchy/Network Process. RACSAM (Review of the Royal spanish Academy of Sciences, Series A, Mathematics) 102 (2): 251–318. Retrieved 2008-12-22.
- Bhushan, Navneet; Kanwal Rai (January 2004). Strategic Decision Making: Applying the Analytic Hierarchy Process. London: Springer-Verlag. ISBN 1-85233-756-7.
- Forman, Ernest H.; Saul I. Gass (2001-07). The analytical hierarchy processan exposition. Operations Research 49 (4): 469–487. doi:10.1287/opre.49.4.469.11231.
 "
"