Team:Paris-Saclay/Project/Notebook/Week 10
From 2012.igem.org
Revision as of 20:54, 25 September 2012 by YohannPetiot (Talk | contribs)
6th August
- Sending of the B1 sample to sequencing with two pairs of primers
- K-274100 Forward and Reverse
- Plasmid pSB1A2 Forward and Reverse
7th August
- Liquid culture of B1 in order to prepare a glycerol stock
- Receipt of new primers
- 2 Forward
- 3 Reverse
- Plasmid Reverse
8th August
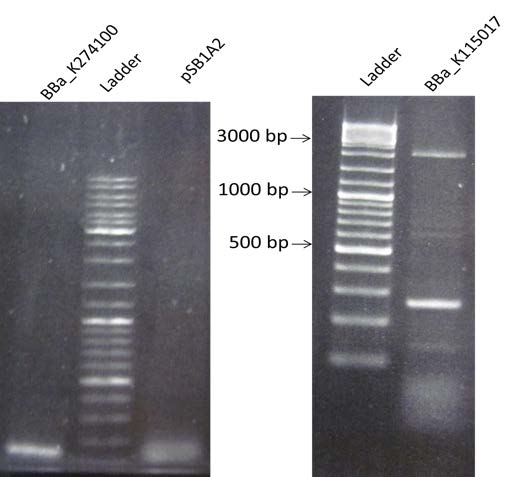
| Amplification of the plasmid pSB1A2, BBa_K274100 and BBa_K115017 by PCR with the new primers. Visualization by electrophoresis on a 2% Agarose gel for BBa_K115017 and a 0.8% Agarose gel for BBa_K274100 and the plasmid pSB1A2. We are expecting a band at 147 bp for BBa_K115017, 3408 bp for BBa_K274100 and 2079 for the plasmid. |
9th August
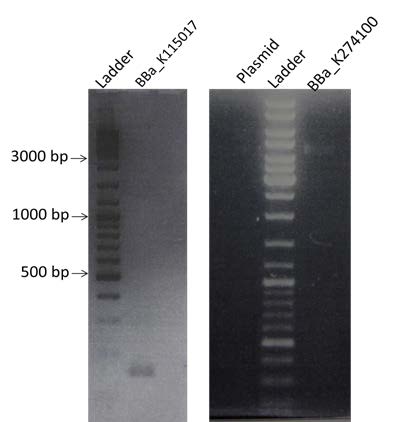
| New amplification by PCR of the plasmid pSB1A2, BBa_K274100 and BBa_K115017 as it has been done the day before. |
- Digestion by EcoRI of the plasmid that contains BBa_K274100.
10th August
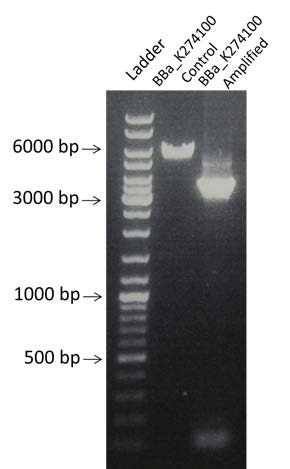
| Amplification by PCR of BBa_K274100 already digested by EcoRI. Visualization by electrophoresis on a 0.8% Agarose gel. |
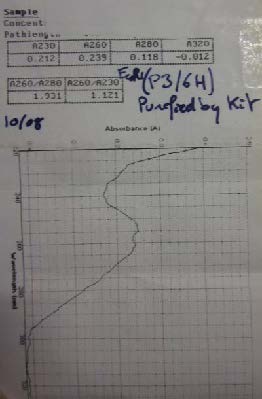
| Miniprep of BBa_K274100 followed by Nanovue to determine the concentration of the sample | |
| Miniprep of the Plasmid pSB1A2 followed by Nanovue to determine the concentration of the sample |
- Digestion by HindIII of the plasmid pSB1A2 in order to linearize it.
| Digestion of BBa_K115017 by DPNI to eliminate the plasmid matrix. Visualization by electrophoresis on a 2% Agarose gel. We are expecting a band at 123 bp. |
 "
"