Team:Cambridge/Ribosense/Labbook
From 2012.igem.org
(→Week 7) |
(→Week 7) |
||
| Line 401: | Line 401: | ||
*Fluoride of concentrations 0.5 - 30 mM mixed with bacterial culture (Yale construct containing) grown up overnight. | *Fluoride of concentrations 0.5 - 30 mM mixed with bacterial culture (Yale construct containing) grown up overnight. | ||
| + | |||
| + | ===Saturday (11/08/12)=== | ||
| + | |||
| + | |||
| + | |||
| + | '''[[Team:Cambridge/Protocols/RestrictionDigest|Restriction digest of Yale plasmid]]''' | ||
| + | |||
| + | ---- | ||
| + | |||
| + | *Cells from electroporation of e.coli with plasmid from Yale (08/08/12) [[Team:Cambridge/Protocols/MiniPrep|miniprepped]] to extract plasmid DNA. | ||
| + | |||
| + | *DNA digested with XhoI and SalI, expected fragment sizes 5580, 3139, 1437 | ||
| + | |||
| + | *Gel run, results not shown as gel was very 'messy'. Gel had probably already begun to set when poured and so had many artefacts when imaged. As a result each lane required different brightness and contrast settings to visualise and so the full gel was not clearly visible on the printout. However, all of the bands could be identified on the screen and were exactly as expected and very clear and pure. | ||
| + | |||
| + | *Bands in correct places, indicating that expected plasmid was present in the cells. This indicates that, although they work at a very low efficiency, our electro-competent cells still work. It may be valuable to use these when trying to grow up plasmids that we already have at high concentration. | ||
| + | |||
| + | '''[[Team:Cambridge/Protocols/TransformationofE.coli|Transformation of e.coli with Magnesium riboswitch construct]]''' | ||
| + | |||
| + | ---- | ||
| + | |||
| + | *Gibson products from 07/08/12 transformed into chemically competent cells. | ||
| + | |||
| + | *Transformants plated out on 100 μg/ml ampicillin plates | ||
==Week 8== | ==Week 8== | ||
Revision as of 03:16, 27 October 2012
Contents |
Judging Form
- Please help the judges by filling out this form. Tell them what medal you think you deserve and why. Tell them which special prizes you should win. Help them find your best parts. Show them how you thought about the safety of your project. Helping the judges will help you too.
- Team: Cambridge
- Region: Europe
- iGEM Year:2012
- Track:Foundational Advance
- Project Name:Parts for a reliable and field ready biosensing platform
- Project Abstract: Implementation of biosensors in real world situations has been made difficult by the unpredictable and non-quantified outputs of existing solutions, as well as a lack of appropriate storage, distribution and utilization systems. This leaves a large gap between a simple, functional sensing mechanism and a fully realised product that can be used in the field.
We aim to bridge this gap at all points by developing a standardised ratiometric luciferase output in a Bacillus chassis. This output can be linked up with prototyped instrumentation and software for obtaining reliable quantified results. Additionally, we have reduced the specialized requirements for the storage and distribution of our bacteria by using Bacillus' sporulation system. To improve the performance of our biosensing platform we have genetically modified Bacillus’ germination speed. Lastly, we demonstrated the robustness of our system by testing it with a new fluoride riboswitch, providing the opportunity to tackle real life problems.
iGEM Medals for non-software teams
- We believe our team deserves the following medal:
- Bronze
- Silver
- √Gold
Because we met the following criteria (check all that apply and provide details where needed)
Requirements for a Bronze Medal
- √Register the team, have a great summer, and plan to have fun at the Regional Jamboree.
- √Successfully complete and submit this iGEM 2012 Judging form.
- √Create and share a Description of the team's project using the iGEM wiki and the team's parts using the Registry of Standard Biological Parts.
- √Plan to present a Poster and Talk at the iGEM Jamboree.
- √Enter information detailing at least one new standard BioBrick Part or Device in the Registry of Standard Biological Parts. Including:
- √Primary nucleaic acid sequence
- √Description of function
- √Authorship
- Safety notes, if relevant.
- √Acknowedgment of sources and references
- √Submit DNA for at least one new BioBrick Part or Device to the Registry.
Additional Requirements for a Silver Medal
- √Demonstrate that at least one new BioBrick Part or Device of your own design and construction works as expected; characterize the operation of your new part/device.
- √Enter this information and other documentation on the part's 'Main Page' section of the Registry
Part Number(s): [http://partsregistry.org/Part:BBa_K911004 BBa_K911004]
Additional Requirements for a Gold Medal: (one OR more)
- Improve an existing BioBrick Part or Device and enter this information back on the Experience Page of the Registry.
Part Number(s): None - √Help another iGEM team by, for example, characterizing a part, debugging a construct, or modeling or simulating their system.
Link to this information on your wiki. Page name: Team:Cambridge/Outreach/Collaboration - √Outline and detail a new approach to an issue of Human Practice in synthetic biology as it relates to your project, such as safety, security, ethics, or ownership, sharing, and innovation.
Link to this information on your wiki.
Page name: Team:Cambridge/HumanPractices/Overview,Team:Cambridge/HumanPractices/MarketResearch,Team:Cambridge/HumanPractices/FutureDirections
iGEM Prizes
All teams are eligible for special prizes at the Jamborees. more... To help the judges, please indicate if you feel you should be evaluated for any of the following special prizes:
- √Best Human Practice Advance
- √Best Experimental Measurement
- Best Model
Please explain briefly why you should receive any of these special prizes:
Best Human Practice Advance:
We feel that we deserve this prize for three reasons:
- We explored the impacts, *both positive and negative*, of synthetic biology as a solution to real world problems, through interviewing professionals working in a relevant field, namely the impact of arsenic water contamination in Bangladesh.
- We recognized existing problems with the way the current direction of synthetic. On going through the registry we found that most of the characterization data for biosensing parts is often neither comparable nor replicable. We have worked to solve this issue, for example with our ratiometric dual channel output.
- *Our project doesn’t stop here*, in Chanel number 6 (Team:Cambridge/HumanPractices/FutureDirections) we considered the future implications and technological applications of our project, as well as the means by which it could be improved by subsequent users. We feel that the end to an iGEM project should not be the conclusion of an idea, but the start of it.
Best BioBrick Measurement Approach:
It is absolutely vital that a quantitative, numerical, robust, and flexible measurement approach exists to relay information to a user that is an accurate representation of the input processed by a biological device. Working from these principles, the following was done:
- We designed and built Biologger, a *cheap, arduino-based, fully functional automatic rotary device* that has an incorporated ratiolumnometer
- Our project is entirely open-sourced and open-platform. We have published source code for the two applications which serve to operate the device, one for PCs and the other for Android devices, as well as the open source circuit design that provides this ratiometric reading. Furthermore, the Android app is able to receive its data wirelessly, which we feel is a great advance in BioBrick measurement.
- Our dual-channel luciferase reporter was successfully tested with a dilution series of E.coli transformed with the Lux Operon (under pBAD) biobrick (Part BBa_K325909) of the Cambridge iGEM 2010 team. It can detect, with good accuracy, both different light intensities, as well as the percentages of blue or orange frequencies in a sample.
- Our device was successfully tested using artificial light to detect different frequencies (colours) as well.
Having done all the above, we believe that this fully open-sourced instrumentation kit (mechanical) chassis, electronics, software code), estimated at *$35.00* (or $85.00 if a Bluetooth modem is required), is a complete BioBrick measurement solution for any and all BioBricks with a light output.
Team_Parts
To help the judges evaluate your parts, please identify 3 of your parts that you feel are best documented and are of the highest quality.
- Best new BioBrick part (natural)
- [http://partsregistry.org/Part:BBa_K911003 BBa_K911003]
- Best new BioBrick part (engineered)
- [http://partsregistry.org/Part:BBa_K911004 BBa_K911004]
- Best improved part(s): None
List any other parts you would like the judges to examine:[http://partsregistry.org/Part:BBa_K911001 BBa_K911001], [http://partsregistry.org/Part:BBa_K911008 BBa_K911009], [http://partsregistry.org/Part:BBa_K911008 BBa_K911008]
Please explain briefly why the judges should examine these other parts:
- Magnesium Sensitive Riboswitch [http://partsregistry.org/Part:BBa_K911001 BBa_K911001]
As a riboswitch sensing construct, this part is an entirely new type of biosensor (along with the fluoride construct) that could potentially change the way we think about designing input genetic circuits. Unlike the fluoride riboswitch, it is a derepression system and therefore serves to demonstrate the principle that riboswitches can be used regardless of whether they turn on or off their reporter. - Fluorescent ratiometric construct for standardizing promoter output [http://partsregistry.org/Part:BBa_K911009 BBa_K911009]
Fluorescence is a major cornerstone for biosensors in the registry, however, most parts do not involve the use of a ratiometric output, which has been shown in the literature to provide much more reliable and meaningful data. This part not only furthers the development of ratiometric measurements in molecular biology but due to the choice of promoters and terminators it can be used to characterize the difference in activity between E. coli and B. Subtilis - Fast Germination (B. subtilis) [http://partsregistry.org/Part:BBa_K911008 BBa_K911008]
This part is entirely novel for the registry and fully utilizes the recombination machinery inherent in the Bacillus chassis. Have spores that can germinate at a faster rate is certainly a worthy achievement and could help with experiments with B. Subtilis that any future iGEM teams may wish to perform.
iGEM Safety
For iGEM 2012 teams are asked to detail how they approached any issues of biological safety associated with their projects.
The iGEM judges expect that you have answered the four safety questions Safety page on your iGEM 2012 wiki.
Please provide the link to that page: Page name: Team:Cambridge/Safety
Attribution and Contributions
For iGEM 2012 the description of each project must clearly attribute work done by the team and distinguish it from work done by others, including the host labs, advisors, and instructors.
Please provide the link to that page, or comments in the box below: Page name: Team:Cambridge/Attributions
Comments
If there is any other information about your project you would like to highlight for the judges, please provide a link to your wiki page here: Team:Cambridge/Overview/DesignProcess
General Labbook
Week 4
Tuesday (17/07/12)
- Plasmid PJS 130 used as our backbone for isolating the magnesium riboswitch.
- Primers:
- Forward: TTCAAAACATGACCTATGACgtcgcagagtatgccg
- Reverse: cctccctctgctaaaacACAAGGCATTAACACTACAT
- PCR settings:
- 95 °C - 6mins
- 98 °C - 10secs
- 58 °C - 45secs
- 72 °C - 180secs
- Repeat above 35x
- 72 °C - 5mins
- 25 °C - 1min
PCR of riboswitch DNA from bacillus genome
- Colony of strain 168 used as template for this experiment.
- Primers used:
- Forward: cggagggagacgattttgTGTTCCGTAATTGTGATGTAAG
- Reverse: acaccggcatactctgcgacGTCATAGGTCATGTTTTGAACC
- PCR settings - as above (run in parallel).
Wednesday (18/07/12)
Gel electrophoresis of PCR products
- PCR fragments for Mg2+ riboswitch from yesterday separated on gel. 90mins at 100V.
- Lane 1: Ladder
- Lane 2 + 3: Riboswitch DNA from genome (replicates) - 550bp fragment expected.
- Lane 4 + 5: Vector DNA from pJS130 (replicates)- Chloramphenicol resistance marker - 9.0kbp fragment expected
- Lane 6: Postitive control - 1.9kbp fragment expected
- Riboswitch DNA was successfully amplified. However, vector DNA was not. Given that the postive control was also successful, it seems likely that something went wrong with some stage before the PCR amplification. We will try to rectify the problem tomorrow.
Purification of riboswitch DNA from gel
- Successful riboswitch DNA extracted from gel and purified using a minelute column. DNA frozen for later Gibson assembly.
Thursday (19/07/12)
- Plasmid PJS 130 used as our backbone for isolating the magnesium riboswitch.
- Primers:
- Forward: TTCAAAACATGACCTATGACgtcgcagagtatgccg
- Reverse: cctccctctgctaaaacACAAGGCATTAACACTACAT
- PCR settings:
- 95 °C - 6mins
- 98 °C - 10secs
- 55 °C - 45secs
- 72 °C - 240secs
- Repeat above 35x
- 72 °C - 5mins
- 25 °C - 1min
- Settings changed - Annealing temperature and elongation time - attempting to debug PCR.
Gel electrophoresis of PCR products
- PCR fragments for Mg2+ riboswitch from yesterday separated on gel. 90mins at 100V.
- Lane 1: Ladder
- Lane 2, 3 + 4: Vector DNA from pJS130 (replicates) - 9.0kbp fragment expected
- Lane 5: Postitive control - 1.9kbp fragment expected
- Lane 6: Negative control - no fragments expected
- Once more, the PCR appears to have failed. We will try to redesign the primers tomorrow to make them functional.
Week 5
Thursday (26/07/12)
PCR of riboswitch DNA from bacillus genome and Lux genes from e.coli genome
- Colony of strain pJS130 used as template for riboswitch, colony of previously transformed e.coli used as template for lux operon.
- M2+ RS
- Forward: cggagggagacgattttg|TGTTCCGTAATTGTGATGTAAG
- Reverse: tataacgttactggtttcat|CGGGACTCGTACCTCC
- Lux operon
- Forward: gctgtacaag|GGAGGAGGAGGAAGTGGAGGAGGAGGAAGT|atgaagtttggaaatatttgtttttc
- Reverse: cctcgcccttgctcaccat|ACTCTATTCCTTTTTGGTGATTC
- PCR settings - as above (run in parallel).
Friday (27/07/12)
Gel electrophoresis of PCR products
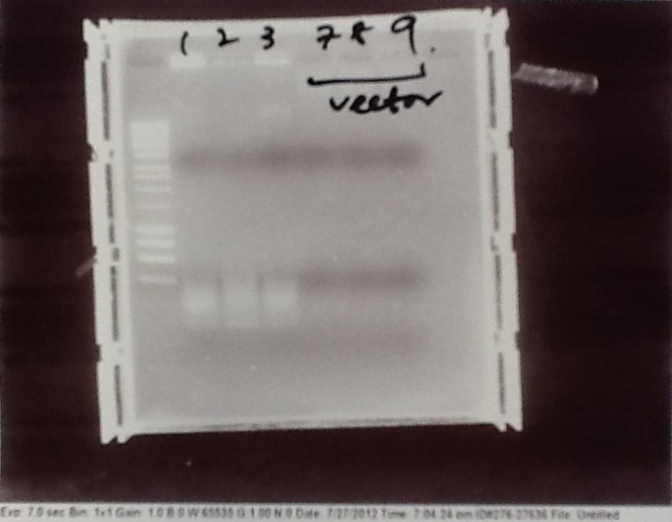
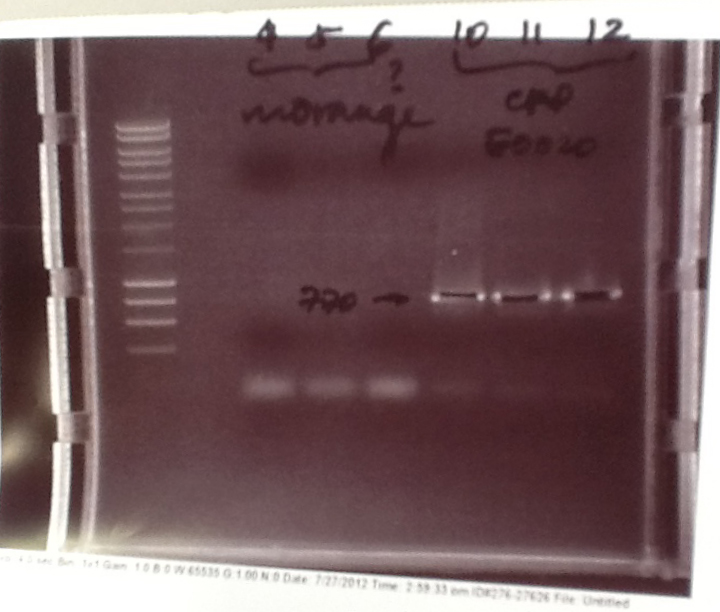
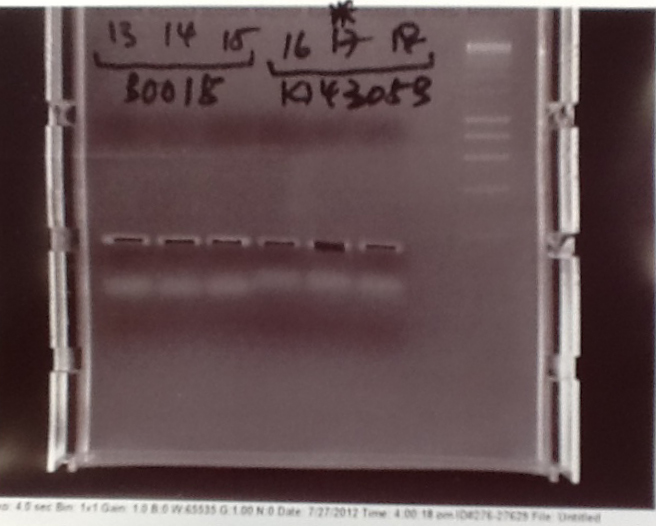
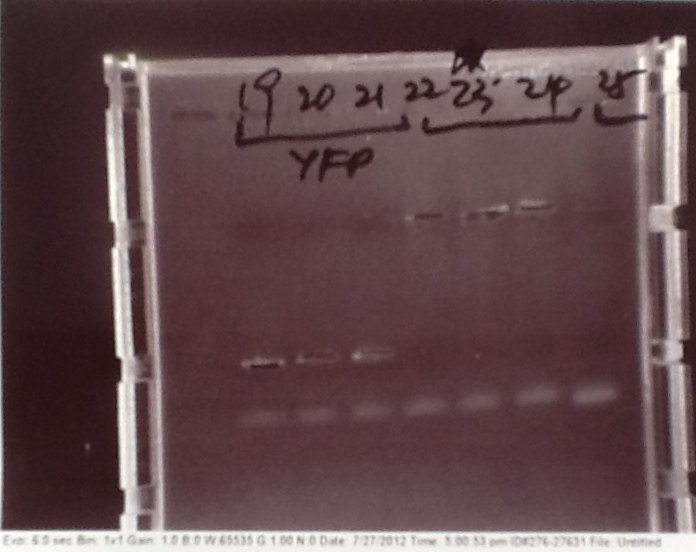
- PCR fragments for Mg2+ riboswitch, luxA/mOrange fusion and fluorescent construct from yesterday separated on gels. 90mins at 100V.
- Mostly successful, but will need to repeat four runs: Three vectors (fluorescent, fusion (lux) and Mg2+ riboswitch (-8 codons) and mOrange gene.
Purification of successfully amplified DNA from gel
- Successful CFP, YFP, B0015, Pveg + SpoVG (K143053), riboswitch and vector DNA extracted from gel and purified using a minelute column. DNA frozen for later Gibson assembly.
Week 6
Monday (30/07/12)
- Not all products were obtained during Friday's PCR. Most of these missing products were large vector backbones. They are being run again, with a much longer extension time of 300s. If that fails, primers will be ordered to split the vectors into manageable chunks, and the PCR reattempted when they arrive.
- PCR cycle x35:
- 15s Denaturing at 95 C
- 45s Annealing at 60 C
- 300s Extension at 72 C
- Remaining stray product had a slightly tricky secondary structure at the 3' end. It will be run at a series of annealing temperatures in a PCR machine capable of a temperature gradient.
- mOrange PCR run at many different temperatures, from 62 °C to 76 °C. However this doesn't seem to solve our problem (refer to Gel photos). It seems likely that it is a primer design problem.
Construction of riboswitch plasmid with Gibson Assembly
- DNA from lanes 27+28, 27+29, 27+30 from gels run on Friday fused together with Gibson assembly to produce riboswitch construct. This does not have replacement of the first 8 codons of lac I with the 8 codons native to the gene downstream of the riboswitch.
- DNA from lanes 22,23 and 24 fused with riboswitch DNA produced two weeks ago to produce riboswitch construct. This has replacement of the first 8 codons of lac I with the 8 codons native to the gene downstream of the riboswitch.
Transformation of Bacillus with riboswitch construct
- Plasmids made by Gibson transformed into bacillus cells made two weeks ago and transformants plated out on 5μg/ml chloramphenicol plates.
Tuesday (01/08/12)
Transformation of Bacillus with riboswitch construct
- Plasmids made by Gibson transformed into bacillus cells made two weeks ago and transformants plated out on 5μg/ml chloramphenicol plates.
Transformation of TOP10 E.coli with riboswitch construct
- Plasmids made by Gibson transformed into TOP10 e.coli cells and transformants plated out on 100μg/ml ampicillin plates.
Wednesday (01/08/12)
Mg2+ Riboswitch
- Successful colonies produced from transformations two days ago streaked out onto chloramphenicol (5 μg/ml) containing plates.
- Colonies also grown up in 10ml of medium A for use with plate reader later.
===Friday (03/08/12)
- PCR of magnesium riboswitch vector repeated with primers to split plasmid.
- Lanes 2 + 3: Fragment A (center - cut site (promotor side))
- Lanes 4 + 5: Fragment B (without 8 codon substitution) (cut site (lac I side) - center)
- Lanes 6 + 7: Fragment B (with 8 codon substitution) (cut stie (lac I side) - center)
- Gels run, found PCR was unsuccessful.
Sunday (05/08/12)
Ribosense: PCR of Mg Riboswitch vector fragments
- 2nd attempt since the PCR on Friday did not work, possibly due to mastermix problems
- PCR Programme:
- Results: Fragment A was successfully PCR-ed (lanes 3-4); Fragment B did not come out, with or without the 8 codons (lanes 5-6 (-8); lanes 7-8 (+8))
Week 7
Monday (06/08/12)
PCR of Magnesium riboswitch vector fragment B and Magnesium promoter
- Normal PCR settings used, annealing temperature 57 °C, elongation step 90s long.
- Lane 5 accidentally loaded with a DNA ladder instead of loading dye.
- Expected fragment sizes:
- Lane 2-5: 3kbp
- Lane 6-7: 300bp
- After electrophoresis, found vector products had, for the most part, worked. Promoter elements were not amplified - no band of the expected size was observed.
- Positive control also failed, although this has ceased to work for several days, potentially due to DNA degradation.
Tuesday (07/08/12)
Gibson assembly of magnesium riboswitch
- NAD+ added to isothermal buffer*5 mix
- Gel slices from yesterday (of vector fragment B) purified.
- DNA added as follows:
- Without 8 codon substitution:
- Reaction 1: Tube 2 (07/08/12) (fragment B), Tube 1 (05/08/12) (fragment A), Tube 28 (29/08/12) (riboswitch DNA).
- Reaction 2: Tube 3 (07/08/12) (fragment B), Tube 2 (05/08/12) (fragment A), Tube 29 (29/08/12) (riboswitch DNA).
- With 8 codon substitution:
- Reaction 3: Tube 4 (07/08/12) (fragment B), Tube 1 (05/08/12) (fragment A), Tube 2 (18/07/12) (riboswitch DNA).
Wednesday (08/08/12)
Electrical transformation of competent e.coli with Gibson products
- Gibson constructs from yesterday (fluorescent and magnesium riboswitch) transformed into e.coli produced yesterday.
- Cells plated out onto 50 μg/ml ampicillin plates. Put in incubator overnight.
Friday (10/08/12)
Characterization of fluoride riboswitch sensitivity with β-galactosidase
- X-Gal made up to a concentration of 400 μg/ml with water.
- Fluoride of concentrations 0.5 - 30 mM mixed with bacterial culture (Yale construct containing) grown up overnight.
Saturday (11/08/12)
Restriction digest of Yale plasmid
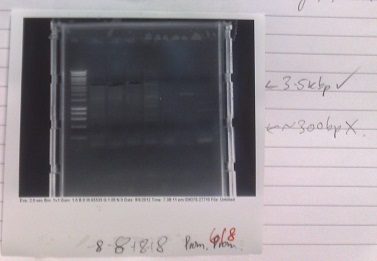
- Cells from electroporation of e.coli with plasmid from Yale (08/08/12) miniprepped to extract plasmid DNA.
- DNA digested with XhoI and SalI, expected fragment sizes 5580, 3139, 1437
- Gel run, results not shown as gel was very 'messy'. Gel had probably already begun to set when poured and so had many artefacts when imaged. As a result each lane required different brightness and contrast settings to visualise and so the full gel was not clearly visible on the printout. However, all of the bands could be identified on the screen and were exactly as expected and very clear and pure.
- Bands in correct places, indicating that expected plasmid was present in the cells. This indicates that, although they work at a very low efficiency, our electro-competent cells still work. It may be valuable to use these when trying to grow up plasmids that we already have at high concentration.
Transformation of e.coli with Magnesium riboswitch construct
- Gibson products from 07/08/12 transformed into chemically competent cells.
- Transformants plated out on 100 μg/ml ampicillin plates
Week 8
Week 9
Week 10
Week 11
Week 12
Week 13
Week 14
 "
"