Team:Tokyo Tech/Experiment/C6
From 2012.igem.org
(→Construction of the 3OC6HSL-dependent 3OC12HSL production module) |
|||
| Line 8: | Line 8: | ||
=Construction of the 3OC6HSL-dependent 3OC12HSL production module= | =Construction of the 3OC6HSL-dependent 3OC12HSL production module= | ||
<div id="tokyotech" style=" font:Arial ;left ; font-size: 15px; color: #000000; padding: 30px;"> | <div id="tokyotech" style=" font:Arial ;left ; font-size: 15px; color: #000000; padding: 30px;"> | ||
| + | |||
For construction of the 3OC6HSL-dependent 3OC12HSL production module, we firstly constructed a new part Plux-LasI ([http://partsregistry.org/wiki/index.php?title=Part:BBa_K934022 BBa_K934022]). Plux-LasI cell is an engineered <I>E.coli</I> that contains a 3OC6HSL-dependent LasI generator and a constitutive LuxR generator. As a 3OC6HSL-dependent LasI generator, we constructed a new Biobrick part Plux-LasI ([http://partsregistry.org/wiki/index.php?title=Part:BBa_K934022 BBa_K934022]) by combining Plux promoter ([http://partsregistry.org/wiki/index.php?title=Part:BBa_R0062 BBa_R0062]) and LasI ([http://partsregistry.org/wiki/index.php?title=Part:BBa_K081016 BBa_K081016] | For construction of the 3OC6HSL-dependent 3OC12HSL production module, we firstly constructed a new part Plux-LasI ([http://partsregistry.org/wiki/index.php?title=Part:BBa_K934022 BBa_K934022]). Plux-LasI cell is an engineered <I>E.coli</I> that contains a 3OC6HSL-dependent LasI generator and a constitutive LuxR generator. As a 3OC6HSL-dependent LasI generator, we constructed a new Biobrick part Plux-LasI ([http://partsregistry.org/wiki/index.php?title=Part:BBa_K934022 BBa_K934022]) by combining Plux promoter ([http://partsregistry.org/wiki/index.php?title=Part:BBa_R0062 BBa_R0062]) and LasI ([http://partsregistry.org/wiki/index.php?title=Part:BBa_K081016 BBa_K081016] | ||
| Line 36: | Line 37: | ||
<br><br> | <br><br> | ||
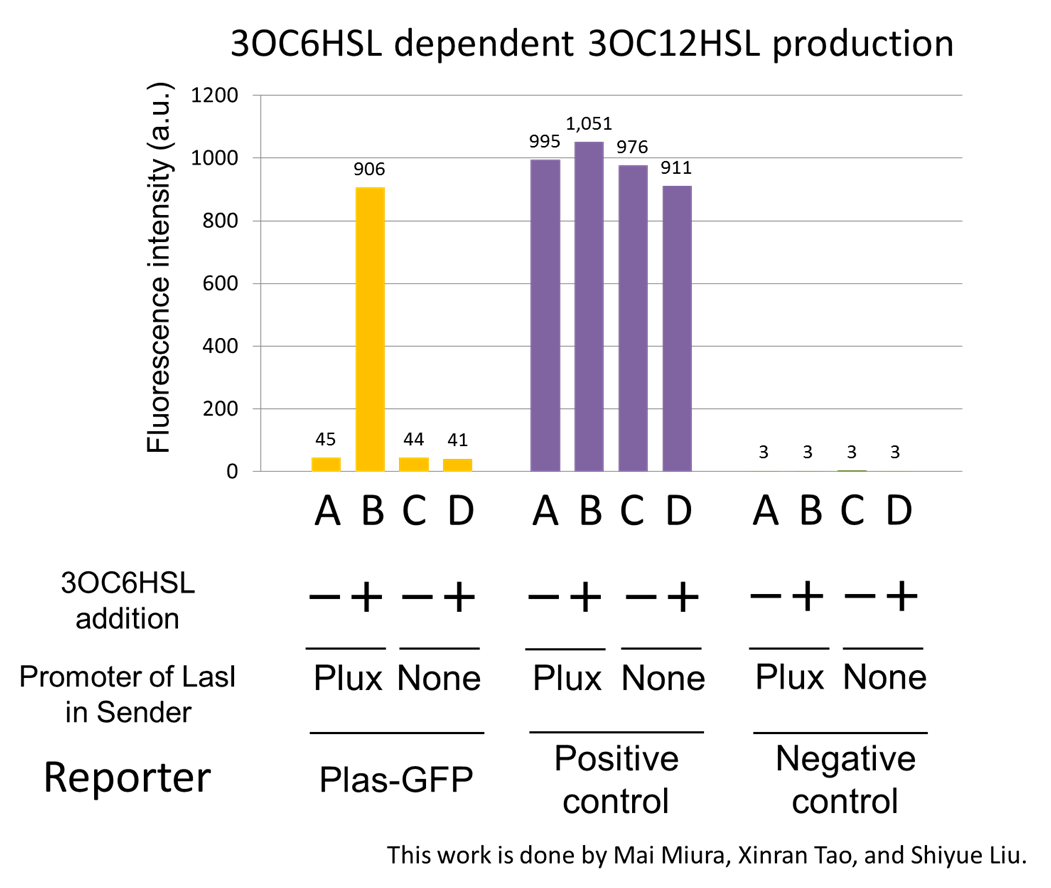
[[File:positivefeedbackassay23tokyotech.png|450px|thumb|right|Fig2-1-3-1-4, 3OC6HSL-dependent 3OC12HSL production]] | [[File:positivefeedbackassay23tokyotech.png|450px|thumb|right|Fig2-1-3-1-4, 3OC6HSL-dependent 3OC12HSL production]] | ||
| - | Fig2-1-3-1-4 shows fluorescence intensities by the reporter cells dependent on different conditions. Only when the supernatant of condition B was used, the fluorescence intensity of the Las reporter cell increased, while the supernatants of other three conditions did not affect. Comparing the results of the condition A and B, it can be said that with the induction of 3OC6HSL to Plux-LasI cell, the fluorescence intensity of the | + | Fig2-1-3-1-4 shows fluorescence intensities by the reporter cells dependent on different conditions. Only when the supernatant of condition B was used, the fluorescence intensity of the Las reporter cell increased, while the supernatants of other three conditions did not affect. Comparing the results of the condition A and B, it can be said that with the induction of 3OC6HSL to Plux-LasI cell, the fluorescence intensity of the Las reporter cell increased by 20-folds. This result indicates that Plux-LasI cell produced 3OC12HSL in response to 3OC6HSL induction by the function of Plux-LasI ([http://partsregistry.org/wiki/index.php?title=Part:BBa_K934022 BBa_K934022] |
). From this experiment, we confirmed that a new part Plux-LasI ([http://partsregistry.org/wiki/index.php?title=Part:BBa_K934022 BBa_K934022] | ). From this experiment, we confirmed that a new part Plux-LasI ([http://partsregistry.org/wiki/index.php?title=Part:BBa_K934022 BBa_K934022] | ||
) synthesized enough concentration of 3OC12HSL to induce the Las reporter cell. | ) synthesized enough concentration of 3OC12HSL to induce the Las reporter cell. | ||
Revision as of 22:49, 26 October 2012
Contents |
Construction of the 3OC6HSL-dependent 3OC12HSL production module
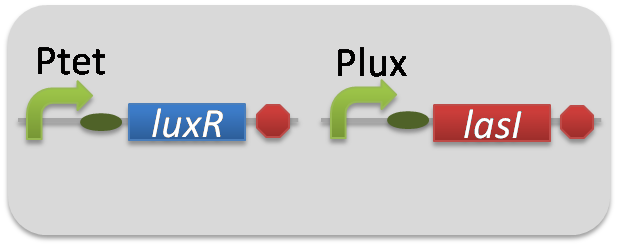
For construction of the 3OC6HSL-dependent 3OC12HSL production module, we firstly constructed a new part Plux-LasI ([http://partsregistry.org/wiki/index.php?title=Part:BBa_K934022 BBa_K934022]). Plux-LasI cell is an engineered E.coli that contains a 3OC6HSL-dependent LasI generator and a constitutive LuxR generator. As a 3OC6HSL-dependent LasI generator, we constructed a new Biobrick part Plux-LasI ([http://partsregistry.org/wiki/index.php?title=Part:BBa_K934022 BBa_K934022]) by combining Plux promoter ([http://partsregistry.org/wiki/index.php?title=Part:BBa_R0062 BBa_R0062]) and LasI ([http://partsregistry.org/wiki/index.php?title=Part:BBa_K081016 BBa_K081016]
). As a constitutive LuxR generator, we used Ptet-LuxR ([http://partsregistry.org/wiki/index.php?title=Part:BBa_S03119 BBa_S03119]). By introducing Plux-LasI ([http://partsregistry.org/wiki/index.php?title=Part:BBa_K934022 BBa_K934022]
) and Ptet-LuxR ([http://partsregistry.org/wiki/index.php?title=Part:BBa_S03119 BBa_S03119]) into E.coli strain JM2.300, we constructed Plux-LasI cell.
Then we performed a reporter assay by using Las reporter cell to characterize the function of Plux-LasI ([http://partsregistry.org/wiki/index.php?title=Part:BBa_K934022 BBa_K934022])
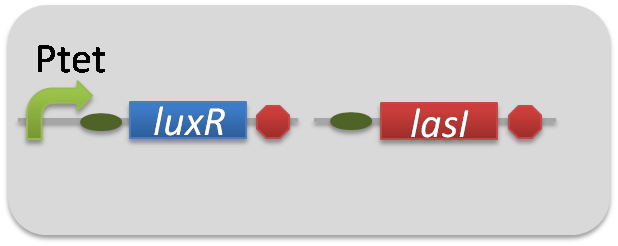
. As the negative control of 3OC12HSL production, we prepared 3OC12HSL non-producer cell (ΔP-LasI cell) that contains, in addition to Ptet-LuxR ([http://partsregistry.org/wiki/index.php?title=Part:BBa_S03119 BBa_S03119]), promoterless-LasI ([http://partsregistry.org/wiki/index.php?title=Part:BBa_K081016 BBa_K081016])
instead of Plux-LasI ([http://partsregistry.org/wiki/index.php?title=Part:BBa_K934022 BBa_K934022]) (Fig2-1-3-1-2).
The ⊿P-LasI cell does not produce 3OC12HSL even though 3OC6HSL exist. The supernatants of the cultures of these modules were used as the inducer in the reporter assay (Fig2-1-3-1-3).
We prepared four conditions as follow.
A) Culture containing Plux-LasI cell without 3OC6HSL induction
B) Culture containing Plux-LasI cell with 3OC6HSL induction
C) Culture containing ⊿P-LasI cell without 3OC6HSL induction
D) Culture containing ⊿P-LasI cell with 3OC6HSL induction
Using the supernatant of the four culture conditions, we performed the reporter assay.
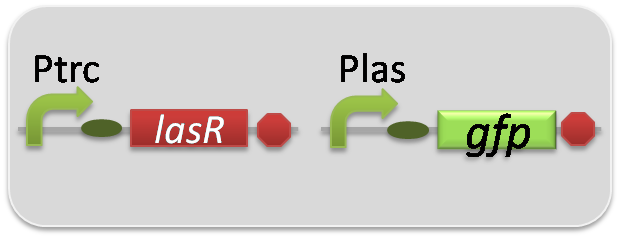
In the reporter assay, we used a Las reporter strain that contains Ptrc-LasR and Plas-GFP ([http://partsregistry.org/wiki/index.php?title=Part:BBa_K649001 BBa_K649001]). Also, a reporter cell that expresses GFP constitutively and a reporter cell that does not express GFP were used as the positive control and the negative control, respectively.
Fig2-1-3-1-4 shows fluorescence intensities by the reporter cells dependent on different conditions. Only when the supernatant of condition B was used, the fluorescence intensity of the Las reporter cell increased, while the supernatants of other three conditions did not affect. Comparing the results of the condition A and B, it can be said that with the induction of 3OC6HSL to Plux-LasI cell, the fluorescence intensity of the Las reporter cell increased by 20-folds. This result indicates that Plux-LasI cell produced 3OC12HSL in response to 3OC6HSL induction by the function of Plux-LasI ([http://partsregistry.org/wiki/index.php?title=Part:BBa_K934022 BBa_K934022]
). From this experiment, we confirmed that a new part Plux-LasI ([http://partsregistry.org/wiki/index.php?title=Part:BBa_K934022 BBa_K934022]
) synthesized enough concentration of 3OC12HSL to induce the Las reporter cell.
Materials & Methods
[Go to the project page "Construction of the 3OC6HSL dependent 3OC12HSL production module"]
1.Construction
pSB6A1-Ptet-LuxR / pSB3K3-Plux-LasI (JM2.300)…Plux-LasI cell
pSB6A1-Ptet-LuxR / pSB3K3-ΔP-LasI (JM2.300)…ΔP-LasI cell
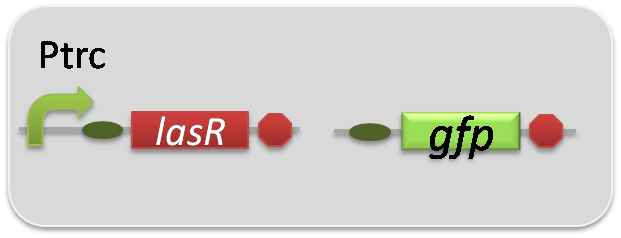
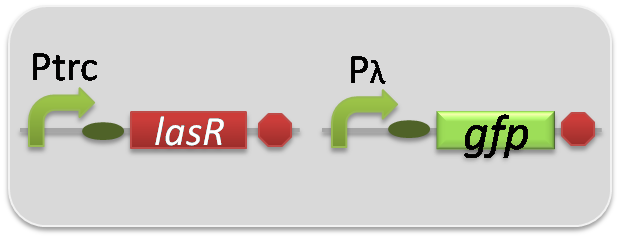
pSB6A1-Ptrc-LasR / pSB3K3-Plas-GFP (JM2.300)…Las reporter cell
pSB6A1-Ptrc-LasR / pSB3K3-ΔP-GFP (JM2.300)…negative control
pSB6A1-Ptrc-LasR / pSB3K3-pλ-GFP (JM2.300)…positive control
2.Strain
JM2.300
Protocol
[Go to the project page "Construction of the 3OC6HSL-dependent 3OC12HSL production module"]
1. collect liquid culture
1.1 Prepare overnight culture of inducer cell at 37°C for 12hours.
1.2 Take 30μl of the overnight culture of inducer cell into LB(3ml) + antibiotics (Amp 50μg/ml + Kan 30μg/ml).(→fresh culture)
1.3 Incubate the flesh culture of inducer cell until the observed OD600 reaches around 0.50. Centrifuge the cell at 5000g, 25°C, 1 min, suspend it with 1ml LB + antibiotics (Amp 50μg/ml).
1.4 Take 30μl cell suspensions into LB(3ml) + antibiotics (Amp 50μg/ml + Kan 30μg/ml) + 5μM 3OC6HSL(3μl) and LB(3ml) + antibiotics (Amp 50μg/ml + Kan 30μg/ml) + DMSO(3μl).
1.5 Incubate the 3OC12HSL producer cells for another 4 hours at 37°C.
1.6 Centrifuge the 3OC12HSL producer cells at 9000g, 4°C, 1 min, and filter the cultured cells.
1.7 Dilute the filtrate by LB + antibiotics (Amp + Kan) in 1:30.
2 Reporter assay
2.1 Prepare overnight culture of reporter cell at 37°C for 12hours.
2.2 Take 30μl of the overnight culture of reporter cell into LB(3ml) + antibiotics (Amp 50μg/ml + Kan 30μg/ml). (→fresh culture)
2.3 Incubate the flesh culture of reporter cell until the observed OD600 reaches around 0.50, and gather the supernatant of culture of inducer cell.
2.4 Centrifuge the reporter cell at 5000g, 25°C, 1 min, and take it into LB + antibiotics (Amp 50μg/ml + Kan 30μg/ml).
2.5 Add 30μl samples of process 2.4 to filtrate + LB(3ml) + antibiotics (Amp 50μg/ml + Kan 30μg/ml) from process 1.7
| 3OC6HSL | Plux |
| + | + |
| - | + |
| + | - |
| - | - |
2.6 Incubate the reporter cells for 4 hours at 37°C.
2.7 Flow cytometer measurements for GFP expression of reporter cells.
 "
"