Team:SJTU-BioX-Shanghai/Project/project2.3
From 2012.igem.org
(→Membrane Accelerator - PAH degradation & DBT desulfurization) |
AleAlejandro (Talk | contribs) (→Membrane Accelerator - PAH degradation & DBT desulfurization) |
||
| Line 56: | Line 56: | ||
We recruited naphthalene degradation pathway in ''Pseudomonas'' species, which has been well characterized. Six crucial enzymes are involved in naphthalene degradation pathway. | We recruited naphthalene degradation pathway in ''Pseudomonas'' species, which has been well characterized. Six crucial enzymes are involved in naphthalene degradation pathway. | ||
| - | [[File:12SJTU_PAHbiodegradation.png|thumb|500px|center|Demonstration of naphthalene degradation pathway in ''Pseudomonas'' species]] | + | [[File:12SJTU_PAHbiodegradation.png|thumb|500px|center|''Fig1:''Demonstration of naphthalene degradation pathway in ''Pseudomonas'' species]] |
In the first catabolic step, an oxygen molecule is introduced at the 1,2-position of the aromatic nucleus to produce cis-1,2-dihydroxy-1,2-dihydronaphthalene by naphthalene dihydrodiol dioxygenase(NahA). cis-1,2-Dihydroxy-1,2-dihydronaphthalene is then dehydrogenated to 1,2-dihydroxynaphthalene by cis-naphthalene dihydrodiol dehydrogenase(NahB). 1,2-Dihydroxynaphthalene is cleaved by 1,2-dihydroxynaphthalene dioxygenase(NahC), and the resulting ring-cleavage product spontaneously cyclizes to form 2-hydroxy-2H-chromene-2-carboxylic acid. Enzymatic reactions by an isomerase(NahD) and a hydratase-aldolase(NahE) result in the production of salicylaldehyde, which is then transformed to salicylate by salicyladehyde dehydrogenase(NahF). | In the first catabolic step, an oxygen molecule is introduced at the 1,2-position of the aromatic nucleus to produce cis-1,2-dihydroxy-1,2-dihydronaphthalene by naphthalene dihydrodiol dioxygenase(NahA). cis-1,2-Dihydroxy-1,2-dihydronaphthalene is then dehydrogenated to 1,2-dihydroxynaphthalene by cis-naphthalene dihydrodiol dehydrogenase(NahB). 1,2-Dihydroxynaphthalene is cleaved by 1,2-dihydroxynaphthalene dioxygenase(NahC), and the resulting ring-cleavage product spontaneously cyclizes to form 2-hydroxy-2H-chromene-2-carboxylic acid. Enzymatic reactions by an isomerase(NahD) and a hydratase-aldolase(NahE) result in the production of salicylaldehyde, which is then transformed to salicylate by salicyladehyde dehydrogenase(NahF). | ||
| Line 62: | Line 62: | ||
To test whether Membrane Accelerator could accelerate naphthalene biodegradation pathway, we are trying to link six crucial enzymes (NahA, B, C, D and E) to orderly organized membrane anchors and expressed them in ''E.coli''. ''E.coli'' expressing the same type and amount of cytoplasmic enzymes is set as control group. | To test whether Membrane Accelerator could accelerate naphthalene biodegradation pathway, we are trying to link six crucial enzymes (NahA, B, C, D and E) to orderly organized membrane anchors and expressed them in ''E.coli''. ''E.coli'' expressing the same type and amount of cytoplasmic enzymes is set as control group. | ||
| - | [[File:12SJTU_PAHconstruction.png|thumb|600px|center|Demonstration of Membrane Accelerator designed for speeding naphthalene biodegradation process]] | + | [[File:12SJTU_PAHconstruction.png|thumb|600px|center|''Fig2:''Demonstration of Membrane Accelerator designed for speeding naphthalene biodegradation process]] |
==Biodesulfurization of Dibenzothiophene (DBT) == | ==Biodesulfurization of Dibenzothiophene (DBT) == | ||
| Line 80: | Line 80: | ||
Note that the first three steps of the this Desulfurization pathway require FMNH<sub>2</sub> as a reductant. In order to regain this power an Oxidoreductase (DszD) uses NADH to recycle the FMNH2, allowing the reaction to proceed. For more information, click [https://2012.igem.org/Team:Calgary/Project/OSCAR/Desulfurization Wiki of team Calgary] | Note that the first three steps of the this Desulfurization pathway require FMNH<sub>2</sub> as a reductant. In order to regain this power an Oxidoreductase (DszD) uses NADH to recycle the FMNH2, allowing the reaction to proceed. For more information, click [https://2012.igem.org/Team:Calgary/Project/OSCAR/Desulfurization Wiki of team Calgary] | ||
| - | [[File:12SJTU desulpathway.png|thumb|700px|center|The 4S Desulfurization Pathway, showing the desulfurization of the model compound DBT by DszA, DszB, DszC, and DszD.]] | + | [[File:12SJTU desulpathway.png|thumb|700px|center|''Fig3:''The 4S Desulfurization Pathway, showing the desulfurization of the model compound DBT by DszA, DszB, DszC, and DszD.]] |
=== Design of Experiment=== | === Design of Experiment=== | ||
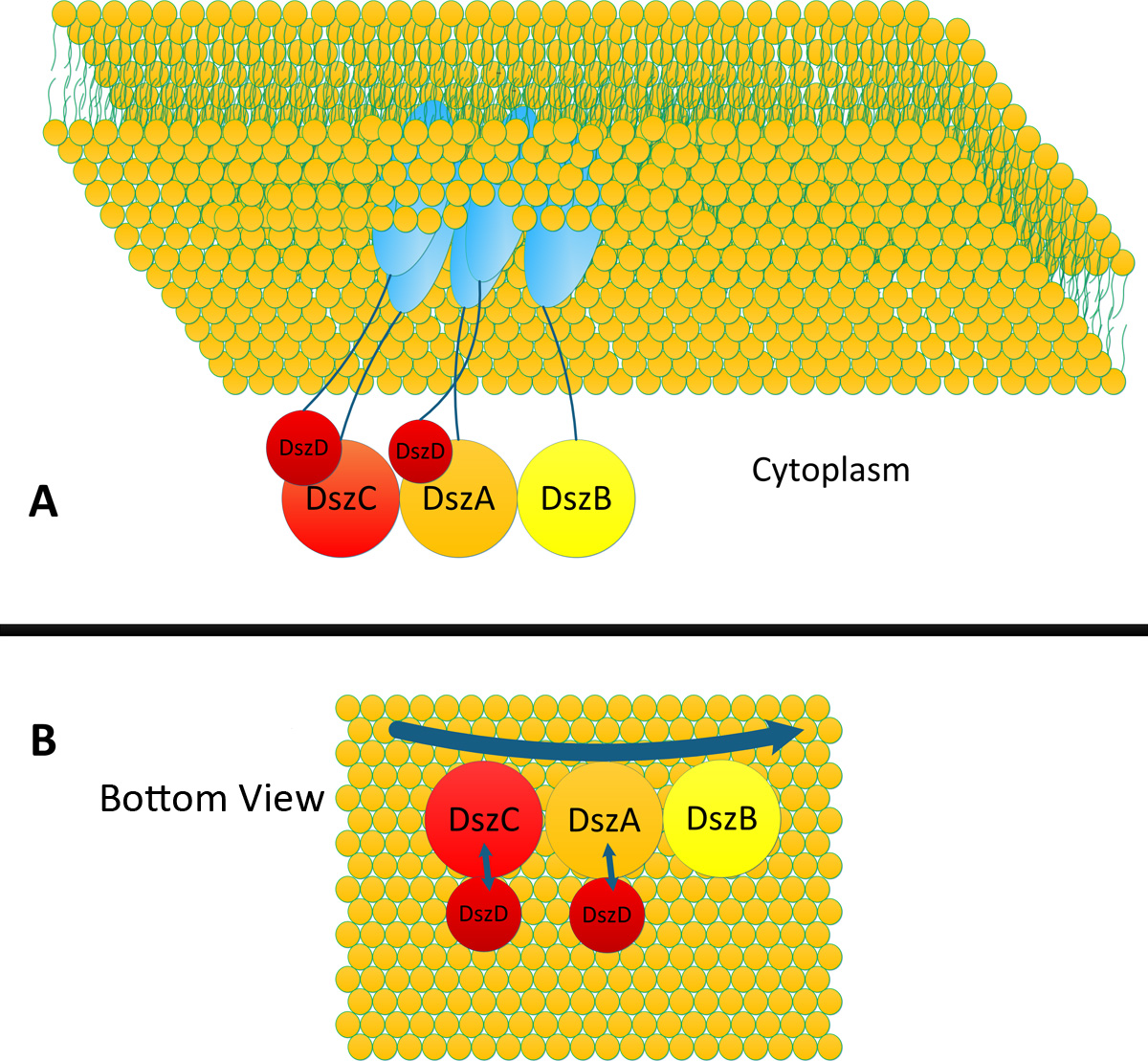
| - | As shown above, DszD plays a special role in this Biodesulfurization pathway: offering FMNH<sub>2</sub> for DszC and DszA. Ideal organization of the four enzymes should be DszC, DszA and DszB aligning together, with DszD paired with DszC and DszA respectively(''Fig.''). In this way, metabolic flux in desulfurization pathway could be facilitated and accelerated. All intermediates could be passed to downstream enzymes efficiently. Furthermore, paired DszD could offer sufficient amount of FMNH<sub>2</sub> for DszC and DszA in time. | + | As shown above, DszD plays a special role in this Biodesulfurization pathway: offering FMNH<sub>2</sub> for DszC and DszA. Ideal organization of the four enzymes should be DszC, DszA and DszB aligning together, with DszD paired with DszC and DszA respectively(''Fig.4''). In this way, metabolic flux in desulfurization pathway could be facilitated and accelerated. All intermediates could be passed to downstream enzymes efficiently. Furthermore, paired DszD could offer sufficient amount of FMNH<sub>2</sub> for DszC and DszA in time. |
| - | [[File:12SJTU desulidealconstruction.png|thumb|600px|center|Ideal organization of DszA, DszB, DszC and DszD in desulfurization pathway. In each assembly two DszD should be paired with DszC and DszA respectively to offer FMNH<sub>2</sub> timely]] | + | [[File:12SJTU desulidealconstruction.png|thumb|600px|center|''Fig4:''Ideal organization of DszA, DszB, DszC and DszD in desulfurization pathway. In each assembly two DszD should be paired with DszC and DszA respectively to offer FMNH<sub>2</sub> timely]] |
This ideal organization of enzymes is hard to achieve with traditional linear synthetic scaffolds. However, Membrane Scaffold is two dimensional, so we can organize corresponding enzymes in desired two dimensional pattern. This again shows the superiority of Membrane Scaffold. | This ideal organization of enzymes is hard to achieve with traditional linear synthetic scaffolds. However, Membrane Scaffold is two dimensional, so we can organize corresponding enzymes in desired two dimensional pattern. This again shows the superiority of Membrane Scaffold. | ||
| - | We are trying to organize DszA, DszB, DszC and DszD according to the pattern shown in '' | + | We are trying to organize DszA, DszB, DszC and DszD according to the pattern shown in ''Fig4''. Due to decreased distance between those enzymes and optimized organization, the proceeding speed of Biodesulfurization pathway should increase sharply. |
| - | [[File:12SJTU desulconstruction.png|thumb|700px|center|Sketch map of Desulfurizing ''Membrane Accelerator'']] | + | [[File:12SJTU desulconstruction.png|thumb|700px|center|''Fig5:''Sketch map of Desulfurizing ''Membrane Accelerator'']] |
==Reference== | ==Reference== | ||
Revision as of 12:54, 26 October 2012
| ||
|
 "
"