Team:SJTU-BioX-Shanghai/Project/project2.2
From 2012.igem.org
AleAlejandro (Talk | contribs) (→The Refinement of Interaction) |
AleAlejandro (Talk | contribs) (→Result and Discussion) |
||
| Line 99: | Line 99: | ||
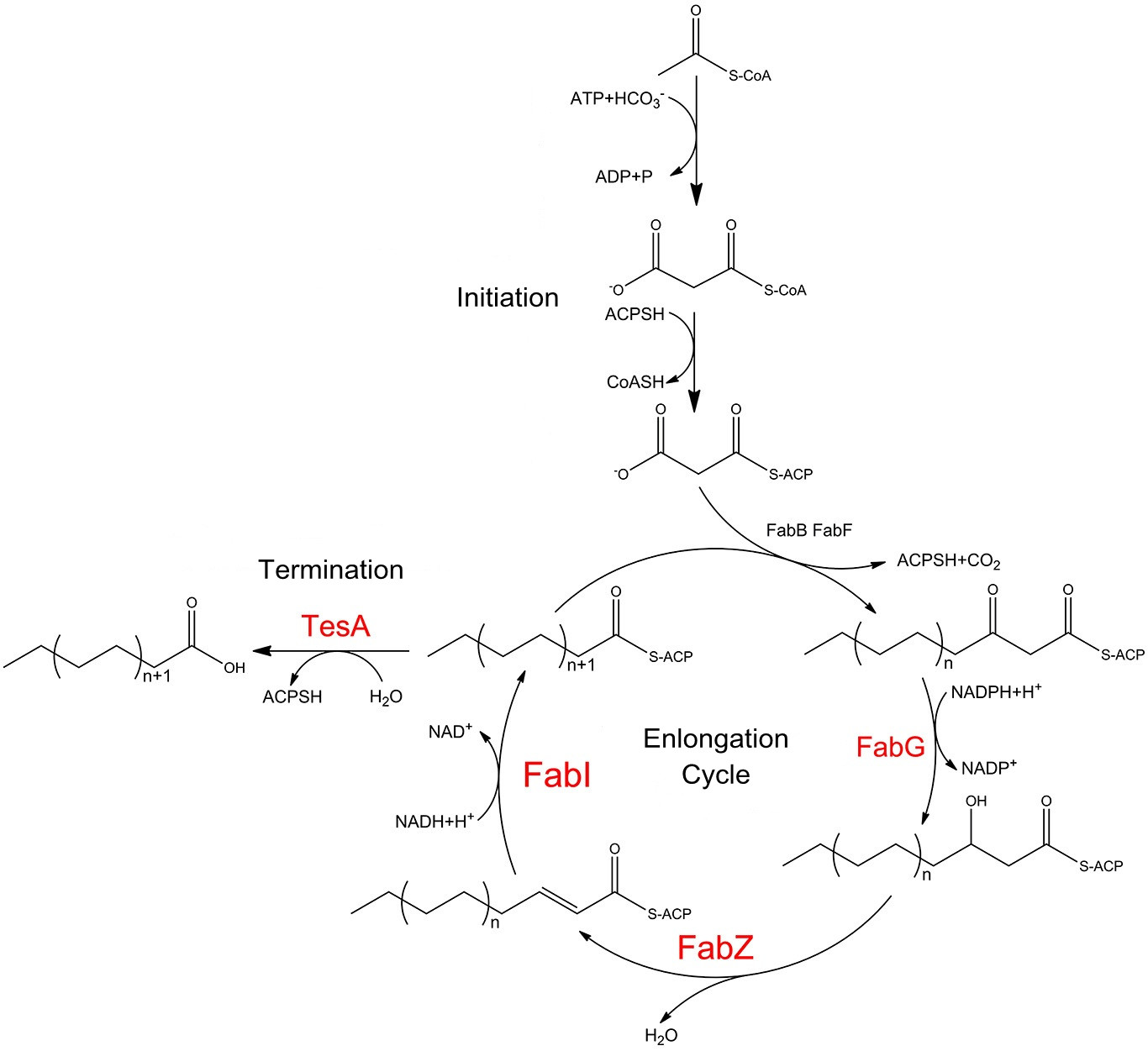
===Assay of the Priority to Exportation=== | ===Assay of the Priority to Exportation=== | ||
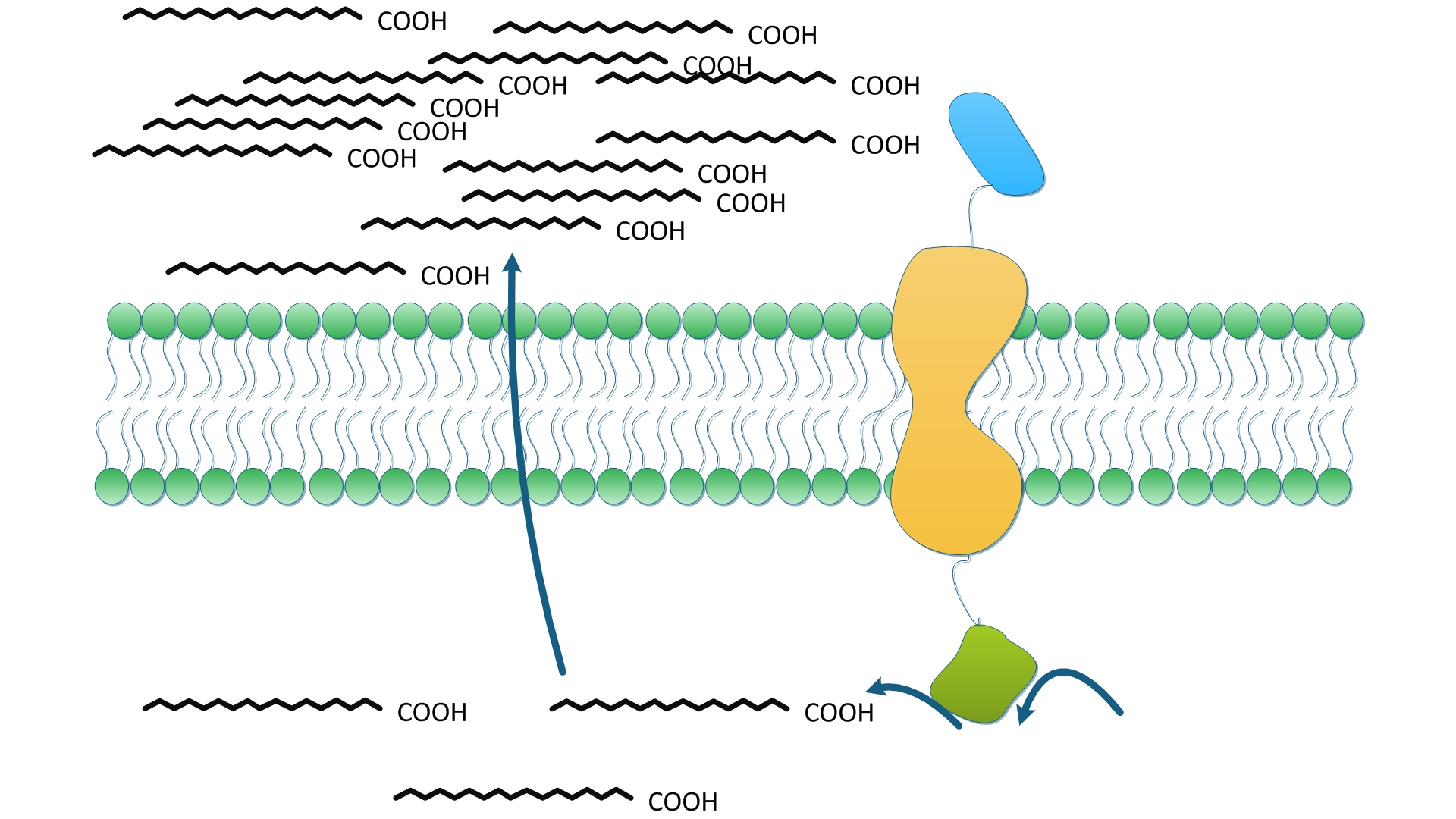
| - | TesA is responsible for the hydrolysis of fatty acyl-ACP, with special affinity to C16- and C18- precursor. As ''Fig. | + | TesA is responsible for the hydrolysis of fatty acyl-ACP, with special affinity to C16- and C18- precursor. As ''Fig.5 A'' indicates, 20 hours after induction, ''E.coli'' with membrane anchored TesA experienced a 50% increase in C-16, C-18 fatty acids content and total fatty acids (''Fig.5 B'') in supernatant, compared with ''E.coli'' with free TesA. |
| - | [[File:12SJTU-TesA-supernatant.png|thumb|center|750px|''Fig. | + | [[File:12SJTU-TesA-supernatant.png|thumb|center|750px|''Fig.5'' shows fatty acid content in supernatant of three groups to evaluate the advantages of membrane anchored TesA. A indicates C16/C18 fatty acids content among three groups. B stands for the total amount of fatty acids among three groups.]] |
Fatty acids yielded from supernatant went up to 0.71mg/(L·OD). The result supports that membrane anchored TesA could efficiently transfer fatty acyl-ACP into desirable fatty acids right beneath inner membrane, thus making it much easier for the final products to diffuse into periplasmic space. | Fatty acids yielded from supernatant went up to 0.71mg/(L·OD). The result supports that membrane anchored TesA could efficiently transfer fatty acyl-ACP into desirable fatty acids right beneath inner membrane, thus making it much easier for the final products to diffuse into periplasmic space. | ||
| - | [[File:12SJTU-TesA-sediment.png|thumb|center|750px|''Fig. | + | [[File:12SJTU-TesA-sediment.png|thumb|center|750px|''Fig.6'' shows fatty acid content in the sediment of three groups to evaluate the advantages of membrane anchored TesA. A indicates C16/C18 fatty acids content among three groups. B stands for the total amount of fatty acids among three groups.]] |
| - | ''Fig. | + | ''Fig.6'', on the other hand, shows fatty acids content in sedimentation also went up by 40%, up to 5.02mg/(L·OD). The result is still within our expectation since fatty acyl-ACP has been removed from the reaction system to form fatty acids. As a result, the chemical equilibrium shifts and more fatty acids would be accumulated. |
We also witnessed a slight decrease in ''E.coli expressing'' free TesA compared with the wildtype, which testifies the statement in a previous study that high levels of TesA inhibits fatty acids synthesis activity but could enhance the activity at low concentrations. | We also witnessed a slight decrease in ''E.coli expressing'' free TesA compared with the wildtype, which testifies the statement in a previous study that high levels of TesA inhibits fatty acids synthesis activity but could enhance the activity at low concentrations. | ||
| Line 116: | Line 116: | ||
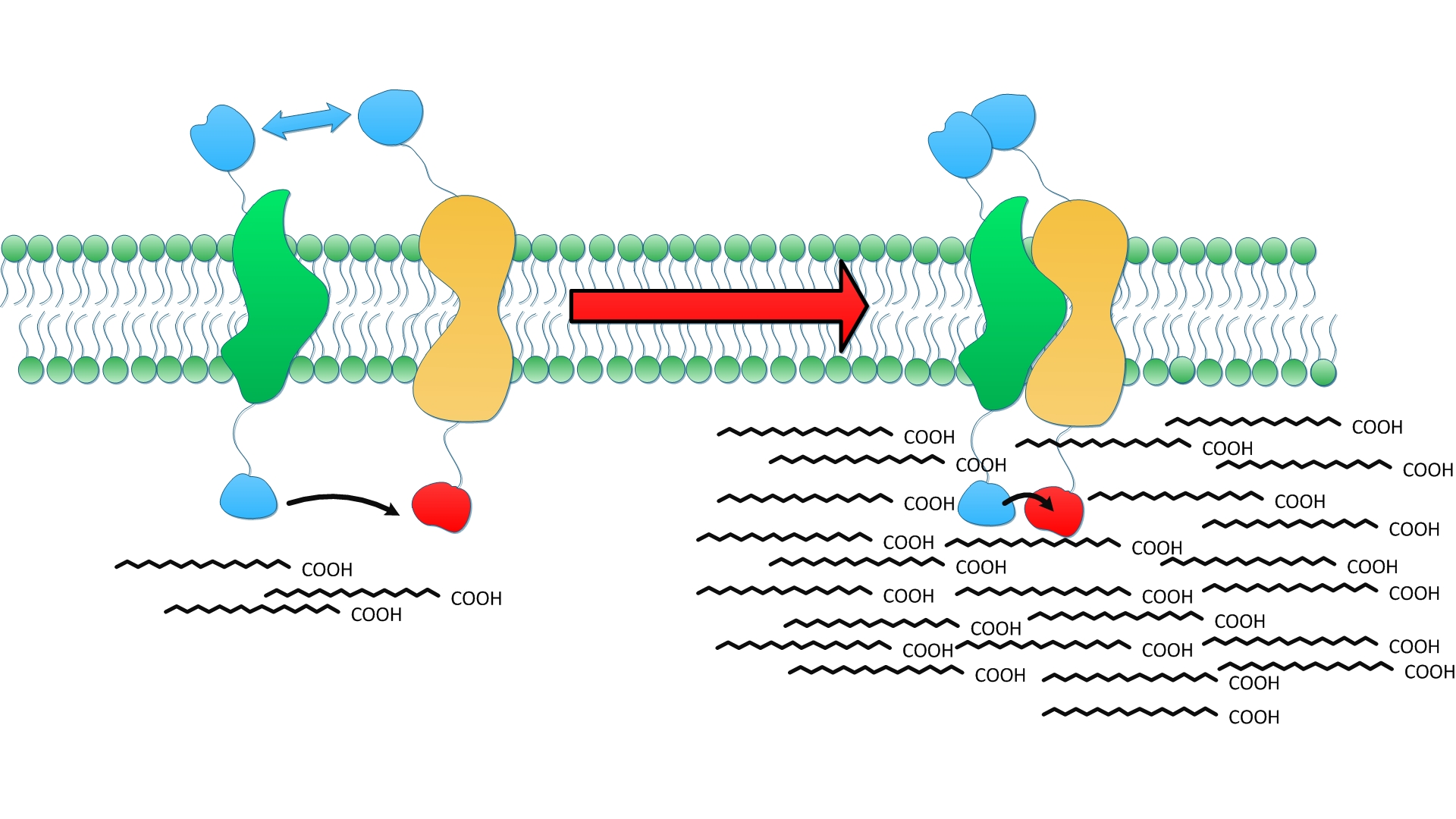
To optimize the productivity of the system we established, we tried to combine these two privileges together, gathering TesA, FabG, FabI and FabZ through protein interaction. Notable increase in both diversity and amount of fatty acids were detected, which lends strong support to our hypothesis. | To optimize the productivity of the system we established, we tried to combine these two privileges together, gathering TesA, FabG, FabI and FabZ through protein interaction. Notable increase in both diversity and amount of fatty acids were detected, which lends strong support to our hypothesis. | ||
| - | [[File:12SJTU-fattyAGZI.png|thumb|center|750px|''Fig. | + | [[File:12SJTU-fattyAGZI.png|thumb|center|750px|''Fig.7'' shows fatty acid content in supernatant of three groups to evaluate the advantages of membrane anchored TesA,FabG,FabZ and FabI. A indicates C16/C18 fatty acids content. B stands for the total amount of fatty acids. C shows the changes in products diversity.]] |
| - | Cluster of FabG, FabI and FabZ provides C16- and C18 specific TesA with sufficient amount of fatty acyl-ACP to hydrolyze and release. Therefore, we witnessed a tremendous growth in the turnover of fatty acids with C16 and C18 skeleton in the supernatant.(Fig. | + | Cluster of FabG, FabI and FabZ provides C16- and C18 specific TesA with sufficient amount of fatty acyl-ACP to hydrolyze and release. Therefore, we witnessed a tremendous growth in the turnover of fatty acids with C16 and C18 skeleton in the supernatant.(Fig.7 A) |
| - | Moreover, fatty acids with C14 skeleton was first detected in ''E.coli'' expressing membrane anchored enzymes compared with ones with free enzymes. It is probably because the productivity of cluster of FabG, FabI and FabZ overloads itself when carbon chain growth slows down as it elongates. Monounsaturated fatty acids also emerge in considerable amount for the first time since TesA is located so closely to the cluster of FabG, FabI and FabZ that it catches intermediate with C16 and C18 skeleton even before they are reduced.(Fig. | + | Moreover, fatty acids with C14 skeleton was first detected in ''E.coli'' expressing membrane anchored enzymes compared with ones with free enzymes. It is probably because the productivity of cluster of FabG, FabI and FabZ overloads itself when carbon chain growth slows down as it elongates. Monounsaturated fatty acids also emerge in considerable amount for the first time since TesA is located so closely to the cluster of FabG, FabI and FabZ that it catches intermediate with C16 and C18 skeleton even before they are reduced.(Fig.7 C) |
| - | Augment in both diversity and amount of fatty acids led to 24 fold increase in total yield compared with wild type and 9 fold with ones expressing free enzymes. (Fig. | + | Augment in both diversity and amount of fatty acids led to 24 fold increase in total yield compared with wild type and 9 fold with ones expressing free enzymes. (Fig.7 B) The exciting result convincingly proves that membrane enhances receptor-ligand interaction and cluster of enzymes makes it faster to a surprising extent. Products acclumulates near inner membrane and traval a shorter distance to diffuse through membrane. |
| - | [[File:12SJTU-123.png|thumb|center|750px|''Fig. | + | [[File:12SJTU-123.png|thumb|center|750px|''Fig.8'' shows fatty acid content in sediment of three groups to evaluate the advantages of membrane anchored TesA,FabG,FabZ,FabI. A indicates C16/C18 fatty acids content. B stands for the total amount of fatty acids.]] |
The fatty acids increase in sediment is relevantly moderate. The reason might be that membrane anchored TesA exports large quantity of fatty acids so the amount remaining in intracellular is limited.C16 and C18 fatty acids in sediment experienced similar trend as that in supernatant and the reason may be the same. It is notable that C19 fatty acids only exist in inside the cell and experience unique changing pattern in which membrane anchored enzymes together produce even less than free ones. We suppose it is caused by the overuse of precursors which inhibits the elongation cycle. Large molecular weight of C19 fatty acids prevent it from exportation and finally they are trapped in the cell. | The fatty acids increase in sediment is relevantly moderate. The reason might be that membrane anchored TesA exports large quantity of fatty acids so the amount remaining in intracellular is limited.C16 and C18 fatty acids in sediment experienced similar trend as that in supernatant and the reason may be the same. It is notable that C19 fatty acids only exist in inside the cell and experience unique changing pattern in which membrane anchored enzymes together produce even less than free ones. We suppose it is caused by the overuse of precursors which inhibits the elongation cycle. Large molecular weight of C19 fatty acids prevent it from exportation and finally they are trapped in the cell. | ||
Revision as of 14:32, 25 October 2012
| ||
|
 "
"