Team:HokkaidoU Japan/Notebook
From 2012.igem.org
| Line 78: | Line 78: | ||
!style="color:blue;"|S | !style="color:blue;"|S | ||
|- | |- | ||
| - | |style="color:red;" | + | |style="color:red;"|1 |
| - | + | ||
| - | + | ||
| - | + | ||
| - | |1 | + | |
|2 | |2 | ||
| - | |style="color:blue;"| | + | |3 |
| + | |4 | ||
| + | |5 | ||
| + | |6 | ||
| + | |style="color:blue;"|7 | ||
|- | |- | ||
| - | |style="color:red;" | + | |style="color:red;"|8 |
| - | + | ||
| - | + | ||
| - | + | ||
| - | |8 | + | |
|9 | |9 | ||
| - | + | |10 | |
| - | + | |11 | |
| - | + | ||
|12 | |12 | ||
|13 | |13 | ||
| - | |14 | + | |style="color:blue;"|14 |
| - | |15 | + | |- |
| + | |style="color:red;"|15 | ||
|16 | |16 | ||
| - | + | |17 | |
| - | + | |18 | |
| - | + | ||
|19 | |19 | ||
|20 | |20 | ||
| - | |21 | + | |style="color:blue;"|21 |
| - | |22 | + | |- |
| + | |style="color:red;"|22 | ||
|23 | |23 | ||
| - | + | |24 | |
| - | + | |25 | |
| - | + | ||
|26 | |26 | ||
|27 | |27 | ||
| - | |28 | + | |style="color:blue;"|28 |
| - | |29 | + | |- |
| + | |style="color:red;"|29 | ||
|30 | |30 | ||
| - | + | |31 | |
| - | + | ||
| | | | ||
|} | |} | ||
Revision as of 09:45, 7 July 2012
| Home | Team | Official Team Profile | Project | Parts Submitted to the Registry | Modeling | Notebook | Safety | Attributions |
|---|
Contents |
Hello
We are team HokkaidoU Japan! Today we learn and start to edit wiki.
(>ω<)
Dear Mr.Ortiz, I saw the help page which you edited.
hola!
March
Spring Boot Camp
- date
- March 5 (Mon) ~ March 9 (Fri)
Monday, March 5
- Session #1
- Short lecture about moleculer biology (Mr.Yamazaki, our adviser)
- Session #2
- Tutrial: How to use 'Unipro UGENE' (iTakeshi)
- Session #3
- Guidance: Wiki Reading (Laury)
- Example: 2010 MIT
Tuesday, March 6
- Session #4~6
- Reading Wikis in turn and discussions
- 2010 NYU
- 2009 Cambridge
- 2009 Growningen
Wednesday, March 7
- Session #7~11
- Reading Wikis (2)
- 2010 Washington
- 2009 Valencia
- 2011 Barklay
- 2010 Paris
- 2010 Bristol
Thursday, March 8
- Session #12
- 2012 Project Brainstorming
- The details is secret! :)
- Session #13
- Guidance: How to read papers (Laury)
Friday, March 9
- Session #14
- 2012 Project Brainstorming (2)
- Session #15
- Guidance: How to look up papers you want (Laury)
- Session #16
- Tutorial: Modeling the behavior of cells (iTakeshi)
- Session #17
- Final Session: Reviewing this camp
- Party!!
Experiment Calender
| July | ||||||
| S | M | T | W | T | F | S |
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 |
| 8 | 9 | 10 | 11 | 12 | 13 | 14 |
| 15 | 16 | 17 | 18 | 19 | 20 | 21 |
| 22 | 23 | 24 | 25 | 26 | 27 | 28 |
| 29 | 30 | 31 | ||||
July
phaABC team
now experimenting...
Ag43&Lysis team
weak 1(4th~10th)
- 4th
- Transformation
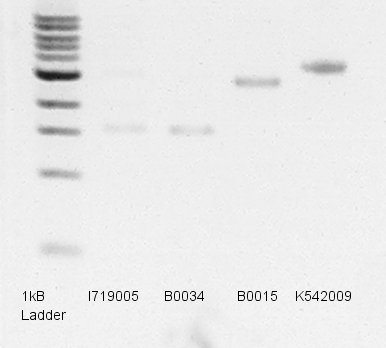
Transformation of BBa_B0015(dT), B0034(RBS), I179005(pT7), K346007(Ag43), and K542009(pLacI-RBS-Ag43) in DH5α
- Added each DNA solutions (1ul) into DH5α comeptent cell and standed on ice in 30 min.
- Cultivated on LBA(dt,RBS,T7) and LBC(Ag43, pLacI-RBS-Ag43). E.coli cultivate in LBC was pre-cultivated in 2 hrs. pT7 was 21hrs and Others were 20hrs cultivated
- 5th
- Transformation
K346007(Ag43) was failed to cultivate on LBC plate. Transformation of K346007(Ag43) in DH5α.
- Add DNA solution(1ul) into DH5α comeptent cell and stand on ice in 30 min.
- Pre-cultivated in 2hrs.
- Cultivated on LBC in 21hrs.
- Single colony isolation
Single colony isolation of BBa_B0015, B0034, I179005 and K542009.
- Picked up one colony.
- Cultivation on LBA(dt,RBS,T7) and LBC(pLacI-RBS-Ag43) in 14hrs30mins
BBa_K542009 was Ag43 only part! And the part didn't have Biobrick suffix.
- 6th
- Liquid culture
Liquid culture in LBA(dT,RBS,pT7) and LBC(pLacI-RBS-Ag43)
- Picked up two colonies from each plates.
- One colony was dipped in 1ml LB (A or C), and other colony was dipped in 2ml LB(A or C). 1ml is for glycerol stocks and 2ml is for mini-prep.
- 16hrs Cultivation
- Single colony isolation
- Single colony isolation of K346007(Ag43).
- 7th
3A assembly! Assembled pT7, RBS and pSB1C3 by 3A assembly. This 3A assembly is our first try!
- mini-prep
- mini-prep of dT,RBS,pT7 and pLacI-RBS-Ag43.
- Elution in 50ul buffer
- Glycerol stock
Made glycerol stocks of dT,RBS,pT7 and pLacI-RBS-Ag43.
- Parts written above were cultivated in LBA(dT,RBS,pT7) and LBC(pLacI-RBS-Ag43) 1ml in 16hrs 30min.
- Add glycerol and Freeze at -80C
- Electrophoresis
Electrophoresis to predict concentration of mini-prep products(dT,RBS,pT7 and pLacI-RBS-Ag43).
- Used 1% agarose gel.
- Pre-migration.
- Migrated 1.2ul of DNA solutions (1ul is mini-prep products and 0.2ul is Loading Dye) in 35min.
- Took a photograph of 1% agarose gel that finished electrophoresis.
- Digestion
Digestion of I719005, B0034 and pSB1K3
Digestion recipe
All parts were reacted in 30ul solution.
- I719005(40ng/ul)
| DNA solution | 12.5ul |
| EcoRI | 1ul |
| SpeI | 1ul |
| 10xH Buffer | 3ul |
| DW | 12.5ul |
- B0034(40ng/ul)
| DNA solution | 12.5ul |
| XbaI | 1ul |
| PstI | 1ul |
| 10xM Buffer | 3ul |
| DW | 12.5ul |
- pSB1K3(25ng/ul)
| DNA solution | 12ul |
| EcoRI | 1ul |
| PstI | 1ul |
| 10xH Buffer | 3ul |
| DW | 13ul |
- Ethanol precipitation
For rising concentration of DNA solution which use for Ligation and removing restriction enzyme.
- Added 3ul of NaoAC, 1.5ul of glycogen and 75ul of 100% ethanol.
- Centrifuged in 14000rpm, 30min at 4C.
- Remove supernatant and added 220ul of 70% ethanol.
- Centrifuged in 15000rpm, 15min at 4C.
- Remove supernatant and air drying in room temperature then added 10ul of DW.
- Ligation
- Transformation
 "
"