|
Background
Advance in molecular cloning technology has made it possible for mankind to entitle engineered organisms to different biochemical reactions. However, the speed of those enzymatic reactions is often limited because intermediates produced from upstream enzyme cannot be passed efficiently to downstream enzyme due to spatial obstacles. Thus, synthetic scaffold built to decrease distance between enzymes to speed biochemical reactions is a hot topic with promising application prospect.
Although some progress has been made in fields of reaction acceleration, no one has before succeeded in dynamically controlling direction of the biochemical pathway.
Introduction
In this year, we expanded the definition of scaffold and developed two universal devices called Membrane Accelerator and Membrane Rudder respectively. Together,they made Membrane Magic happen!
Previous researchers have focused on building protein, RNA or DNA scaffold as constitutive assemblies carrying enzymes. They have succeeded in increasing product yields. However, the amount of those scaffolds could be limited by its expression or copy level, leading to restriction on further acceleration. Through Membrane Magic Project, we engineered E.coli membrane into a huge scaffold accommodating enzymes without limitation of scaffold amount. Moreover, protein assembly on membrane could readily receive extracellular or intracellular signal, so the whole system becomes highly tunable, different from previous work.
One of our devices, called Membrane Accelerator, functions by localizing enzymes to membrane surface. E.coli inner membrane serves as a two-dimensional plane that can accommodate different protein assemblies linked with enzymes. Otherwise diffusing enzymes can form clusters on membrane through interacting protein domains and ligands. Enzyme clusters help substrates flow between enzymes, and thus increase yields of sequential biological reactions.

Fig.1: Sketch of Membrane Accelerator
As some work has been done in reaction acceleration, people failed to control those reactions artificially and dynamically during biosynthetic process. Membrane Rudder device, however, offers a novel method to control the direction of reactions through light and chemical signals. More strikingly, we further combine Membrane Rudder system with genetic circuits by recruiting RNA aptamer and its corresponding binding protein. Thus both extracellular and intracellular signal would trigger the subsequent reaction.
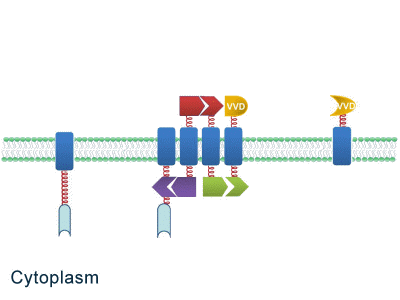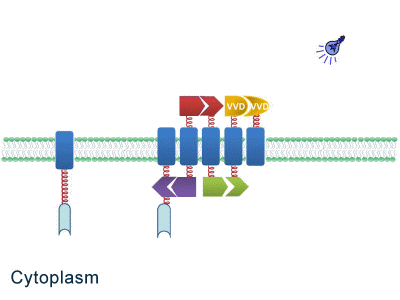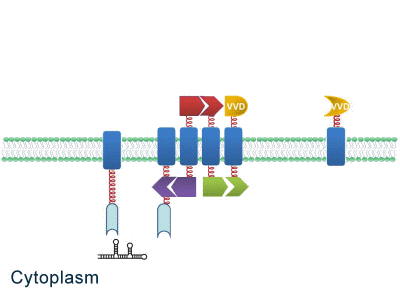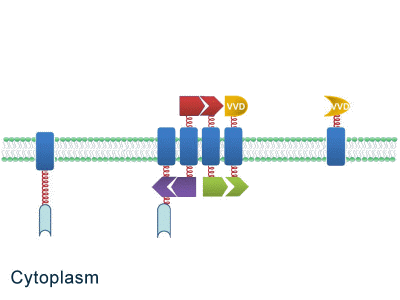
Fig.2: Sketch of Membrane Rudder
Why MEMBRANE?
Why do we choose membrane as our primary scaffold to assemble enzymes?
1.Priority to Exportation: Final products would be more readily to be exported to extracellular media if enzymes are localized to membrane.
2.Two-Dimensional Plane: Our project changes the dimension of traditional reaction space, making possible the assembling of various reactions on a two-dimensional plane, which has been proved to accelerate reaction more sharply than one-dimensional or discrete scaffold.
3.Tendency to Interaction: Previous study has developed discrete protein scaffolds which recruit enzymes diffusing all over cytoplasm. In our study, enzymes were anchored onto the membrane and become more likely to interact with each other.
4.Ability to Sense Signal: Membrane Scaffold provides a platform to receive environmental and internal signal. Thus reactions could be controlled through those signals.
|
 "
"

