Team:HokkaidoU Japan/Notebook/aggregation Week 10
From 2012.igem.org
| Line 277: | Line 277: | ||
#Removed supernatant and added 220 ul of 70% ethanol. | #Removed supernatant and added 220 ul of 70% ethanol. | ||
#Centrifuged at 15000 rpm, 10 min at 4C. | #Centrifuged at 15000 rpm, 10 min at 4C. | ||
| - | #Removed supernatant and | + | #Removed supernatant and dried out at room temperature, after that added 10 ul of DW. |
| Line 598: | Line 598: | ||
#Removed supernatant and added 220 ul of 70% ethanol. | #Removed supernatant and added 220 ul of 70% ethanol. | ||
#Centrifuged at 15000 rpm, 15 min at 4C. | #Centrifuged at 15000 rpm, 15 min at 4C. | ||
| - | #Removed supernatant and | + | #Removed supernatant and dried out at room temperature, after that added 10 ul of DW. |
| Line 654: | Line 654: | ||
#Plated 300 ul of the culture onto first dish and spread. | #Plated 300 ul of the culture onto first dish and spread. | ||
#Mixed 450 ul of LB to 50 ul of the culture and plated 300 ul of it onto second dish and spread. | #Mixed 450 ul of LB to 50 ul of the culture and plated 300 ul of it onto second dish and spread. | ||
| - | #Incubated the plates at 37C for 19 | + | #Incubated the plates at 37C for 19 hrs. |
</p> | </p> | ||
| Line 739: | Line 739: | ||
#Plated 300 ul of the culture onto first dish and spread. | #Plated 300 ul of the culture onto first dish and spread. | ||
#Mixed 450 ul of LB to 50 ul of the culture and plated 300 ul of it onto second dish and spread. | #Mixed 450 ul of LB to 50 ul of the culture and plated 300 ul of it onto second dish and spread. | ||
| - | #Incubated the plates at 37C for 19 | + | #Incubated the plates at 37C for 19 hrs. |
</p> | </p> | ||
</div></div> | </div></div> | ||
Revision as of 21:30, 26 September 2012
September 3rd
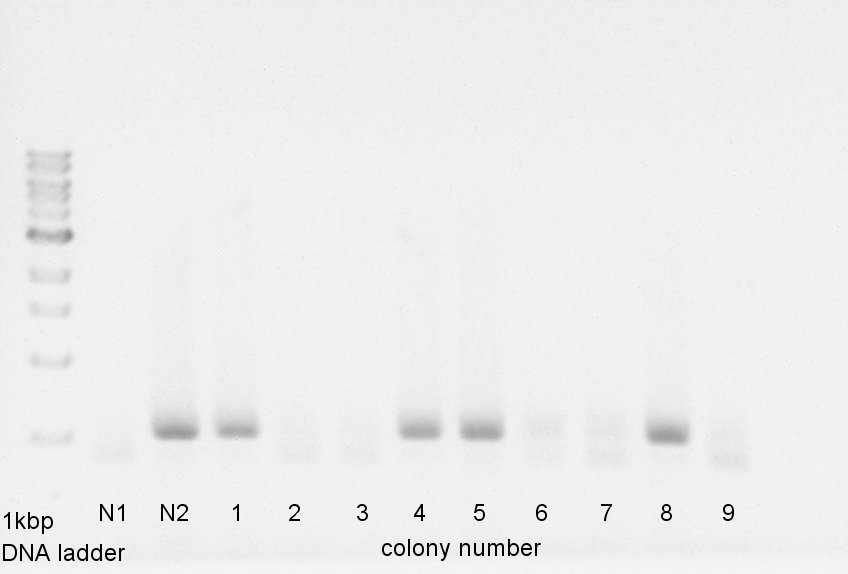
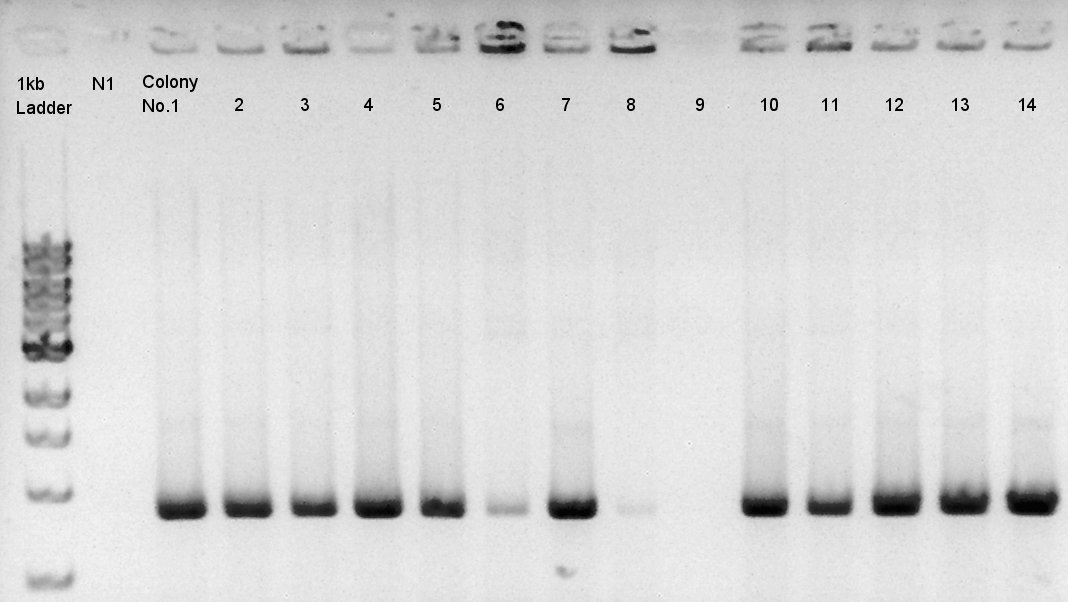
Colony PCR
Colony PCR to confirm that whether the pBAD-RBS-Ag43-dT was successfully ligated with pSB1C3 or not.
| DNA solution | 4 ul |
| Kapa-Taq(Taq polymerase) | 10 ul |
| Forward Primer(Ag43-f4) | 1 ul |
| Reverse Primer(PS-Reverse) | 1 ul |
| DW | 4 ul |
| Total | 20 ul |
| Number | Degree | Second |
| 1 | 95 | 120 |
| 2 | 95 | 30 |
| 3 | 53.2 | 30 |
| 4 | 72 | 60 |
| 5 | 72 | 60 |
| 6 | 4 | HOLD |
Cycle:2~4 x 35
We used N1 (DW only) and N2 (pBAD-RBS-Ag43-dT on pSB1Ak3) as controls. Desired product is about 600bp.
Colony PCR of eCFP-RBS-pSB1A2
| DNA solution | 4 ul |
| Kapa-Taq(Taq polymerase) | 5 ul |
| Forward Primer(EX-F primer) | 0.5 ul |
| Reverse Primer(PS-R down primer) | 0.5 ul |
| Total | 10 ul |
| Number | Degree | Second |
| 1 | 95 | 120 |
| 2 | 95 | 30 |
| 3 | 68.9 | 30 |
| 4 | 72 | 60 |
| 5 | 72 | 60 |
| 6 | 4 | HOLD |
Cycle:2~4 x 35
We used N1 (DW only) and N2 (ptetR-RBS-eCFP-dT-pSB1A2) as controls. Desired product is about 776bp.
We confirmed that about 70% of ligated DNA formed our desired construct. We selected No. 2 and 5 colony for incubation and store No. 7 and 8 colony mixture at 4C.
Incubation of eCFP-RBS-pSB1A2 for plasmid extraction
- Prepared 2 ml LBA into culture tubes.
- Re-suspended 2 colony mixture (No. 2 and No. 5 respectively).
- Incubated at 37C for 17 hrs.
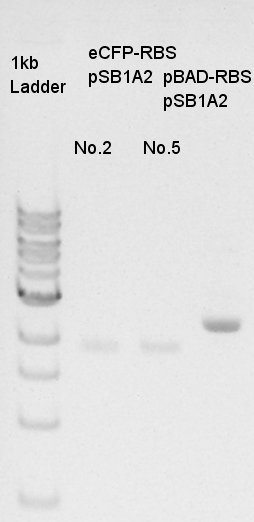
Estimation of concentration of eCFP-RBS-pSB1A2 and pBAD-RBS-pSB1A2
No. 2,5 means the colony number of colony PCR of eCFP-RBS-pSB1A2.
We estimated the concentration of eCFP-RBS-pSB1A2 is 30 ng/ul and pBAD-RBS-pSB1A2 is 50 ng/ul.
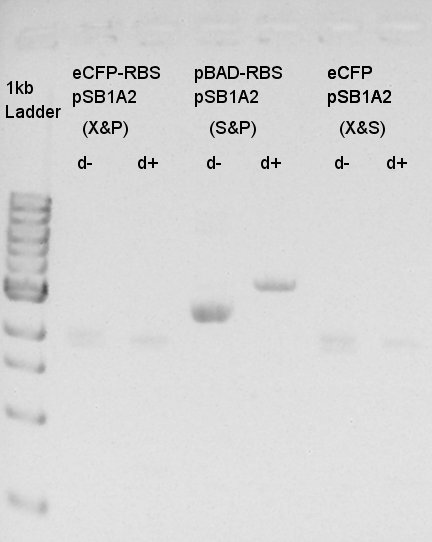
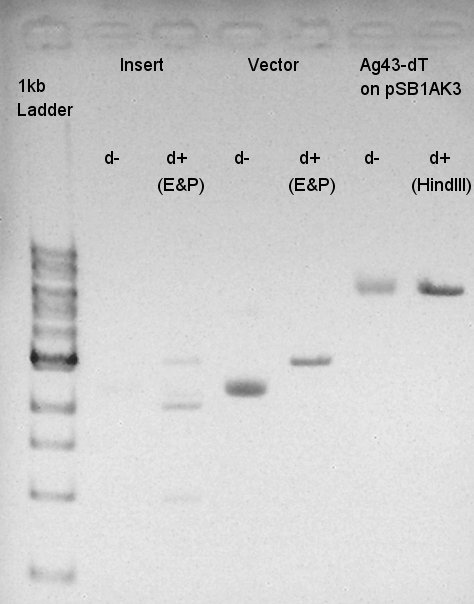
Digestion of eCFP-RBS-pSB1A2 and pBAD-RBS-pSB1A2
To make a construct of pBAD-RBS-eCFP-RBS-pSB1A2, we digested eCFP-RBS-pSB1A2 with XbaI & PstI and pBAD-RBS-pSB1A2 with SpeI and PstI. And we digested eCFP-pSB1A2 with XbaI and SpeI as a control for confirmation of the ability to digest. Insert (eCFP-RBS-pSB1A2)
| DNA solution (30 ng/ul) | 22 ul |
| XbaI | 1 ul |
| PstI | 1 ul |
| 10xM buffer | 3 ul |
| DW | 3 ul |
| Total | 30 ul |
Vector(pBAD-RBS-pSB1A2)
| DNA solution (50 ng/ul) | 3 ul |
| SpeI | 1 ul |
| PstI | 1 ul |
| 10xH buffer | 2 ul |
| DW | 13 ul |
| Total | 20 ul |
control (eCFP-pSB1A2)
| DNA solution (30 ng/ul) | 5 ul |
| XbaI | 1 ul |
| SpeI | 1 ul |
| 10xM buffer | 2 ul |
| DW | 11 ul |
| Total | 20 ul |
| Number | Degree | Minute |
| 1 | 37 | 120 |
| 2 | 60 | 15 |
| 3 | 4 | HOLD |
From this image, we confirmed that DNA were digested into fragments and all of restriction enzyme worked.
September 4th
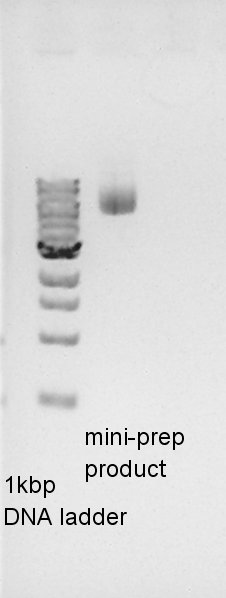
Plasmid extraction
Plasmid extraction of pBAD-RBS-Ag43-dT on pSB1C3. We used plasmid extraction kit of Nippon genetics: FastGene Plasmid mini kit and finally we eluted the DNA with 50 ul of elution buffer.
Ethanol precipitation of digestion products (eCFP-RBS and pBAD-RBS-pSB1A2) and estimation of concentration
- Added 5 ul of NaoAc, 1.5 ul of glycogen and 125 ul of 100% ethanol.
- Centrifuged at 15000 rpm, 15 min at 4C.
- Removed supernatant and added 220 ul of 70% ethanol.
- Centrifuged at 15000 rpm, 10 min at 4C.
- Removed supernatant and dried out at room temperature, after that added 10 ul of DW.
We estimated the concentration of ethanol presipitation products.The concentration of Insert DNA solution is about 20 ng/ul and Vector DNA solution is about 50 ng/ul.
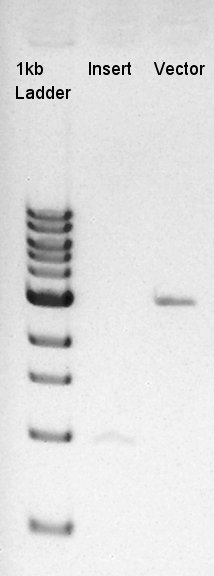
Ligation of eCFP-RBS and pBAD-RBS-pSB1A2
| Vector DNA (50 ng/ul) | 1.5 ul |
| Insert DNA (20 ng/ul) | 4 ul |
| Ligation Mighty Mix | 6 ul |
| DW | 0.5 ul |
| Total | 12 ul |
Ligation reaction time was in detail below.
| Degree | Minute |
| 16 | 30 |
| 65 | 10 |
| 4 | Hold |
Transformation of pBAD-RBS-eCFP-RBS-pSB1A2
- Mixed 2 ul pBAD-RBS-eCFP-RBS-pSB1A2 ligation product to 50 ul of thawed competent cells on ice.
- Incubated on ice for 30 min.
- Mixed 350 ul of LB.
- Prepared and Labeled two plastic plates with LB plate medium which contained appropriate antibiotics (LBA).
- Plated 300 ul of the culture onto first dish and spread.
- Mixed 450 ul of LB to 50 ul of the culture and plated 300 ul of it onto second dish and spread.
- Incubated the plates at 37C for 25 hours.
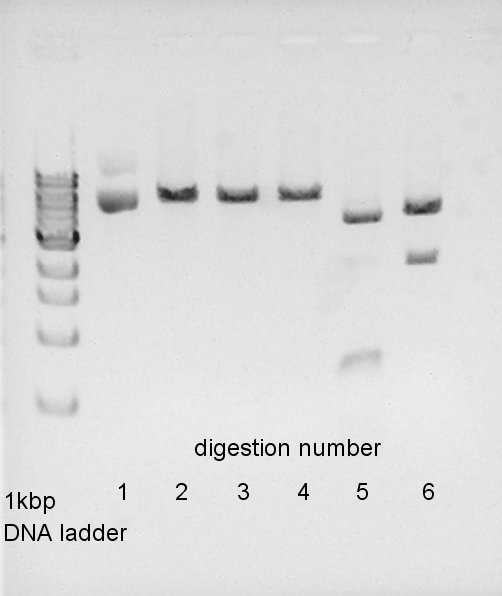
Digestion of ptet-RBS-eYFP-dT-pSB1A2 and pSTV28
To make a construct of ptet-RBS-eYFP-dT-pSTV28, we digested ptet-RBS-eYFP-dT-pSB1A2 with EcoRI and PstI, and pSTV28 with EcoRI and PstI. And we digested Ag43-dT-pSB1AK3 by HindIII. Insert (ptet-RBS-eYFP-dT-pSB1A2)
| DNA solution (30 ng/ul) | 20 ul |
| EcoRI | 1 ul |
| PstI | 1 ul |
| 10xH buffer | 3 ul |
| DW | 5 ul |
| Total | 30 ul |
Vector(pSTV28)
| DNA solution (50 ng/ul) | 3 ul |
| EcoRI | 1 ul |
| PstI | 1 ul |
| 10xH buffer | 2 ul |
| DW | 13 ul |
| Total | 20 ul |
Ag43-dT-pSB1AK3
| DNA solution (30 ng/ul) | 30 ul |
| HindIII | 2 ul |
| 10xM buffer | 5 ul |
| DW | 13 ul |
| Total | 50 ul |
| Number | Degree | Minute |
| 1 | 37 | 120 |
| 2 | 60 | 15 |
| 3 | 4 | HOLD |
We confirmed that ptet-RBS-eYFP-dT-pSB1A2 was partially digested and pSTV28 would be successfully digested we think. But we couldn't confirm whether Ag43-dT-pSB1AK3 was digested or not.
We did a gel-extraction of these digested DNA and got 50ul solution of each DNA.
September 5th
Digestion
We digested plasmid extraction products (pBAD-RBS-Ag43-dT on pSB1AK3) by restriction enzyme. Because I'd like to check the size of insert and vector of getting plasmid. No. 1
| DNA solution | 1 ul |
| EcoRI | 1 ul |
| 10xH buffer | 1 ul |
| DW | 7 ul |
| Total | 10 ul |
No. 2
| DNA solution | 1 ul |
| XbaI | 1 ul |
| 10xM buffer | 1 ul |
| 10xBSA buffer | 1 ul |
| DW | 6 ul |
| Total | 10 ul |
No. 3
| DNA solution | 1 ul |
| SpeI | 1 ul |
| 10xM buffer | 1 ul |
| DW | 7 ul |
| Total | 10 ul |
No. 4
| DNA solution | 1 ul |
| PstI | 1 ul |
| 10xH buffer | 1 ul |
| DW | 7 ul |
| Total | 10 ul |
No. 5
| DNA solution | 2 ul |
| EcoRI | 1 ul |
| SpeI | 1 ul |
| 10xH buffer | 2 ul |
| DW | 14 ul |
| Total | 20 ul |
| Number | Degree | Minute |
| 1 | 37 | 120 |
| 2 | 60 | 15 |
| 3 | 4 | HOLD |
September 6th
Ag43 expressing
We usually used plastic tube for incubating. The E. coli expressing Ag43 attachment to the face of tube wall.
We estimated the reason is attachment to hydrophobic materials. We incubated in 1 ml of LBA (one contained 1% of L-arabinose, another did not contain L-arabinose) at 37C in glass tubes.
Ethanol precipitation of ptet-RBS-eYFP-dT-pSB1A2 and pSTV28
- Added 5 ul of NaoAc, 1.5 ul of glycogen and 125 ul of 100% ethanol.
- Centrifuged at 14000 rpm, 30 min at 4C.
- Removed supernatant and added 220 ul of 70% ethanol.
- Centrifuged at 15000 rpm, 15 min at 4C.
- Removed supernatant and dried out at room temperature, after that added 10 ul of DW.
We confirmed that the concentration of Insert DNA solution is 10~20 ng/ul and Vector DNA solution is 30~40 ng/ul.
Ligation of ptet-RBS-eYFP-dT-pSB1A2 and pSTV28
| Vector DNA (30~40 ng/ul) | 1.5 ul |
| Insert DNA (10~20 ng/ul) | 4 ul |
| Ligation Mighty Mix | 6 ul |
| DW | 0.5 ul |
| Total | 12 ul |
Ligation reaction time was in detail below.
| Degree | Minute |
| 16 | 30 |
| 65 | 10 |
| 4 | Hold |
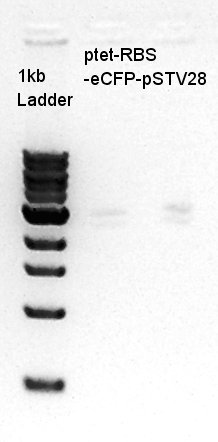
Transformation of ptet-RBS-eYFP-dT-pSTV28
- Mixed 2 ul ptet-RBS-eYFP-dT-pSTV28 ligation product to 50 ul of thawed competent cells on ice.
- Incubated on ice for 30 min.
- Mixed 350 ul of LB.
- Incubated for 2 hrs to get the resistance to Chloramphenicol.
- Prepared and Labeled two plastic plates with LB plate medium which contained appropriate antibiotics (LBC).
- Plated 300 ul of the culture onto first dish and spread.
- Mixed 450 ul of LB to 50 ul of the culture and plated 300 ul of it onto second dish and spread.
- Incubated the plates at 37C for 19 hrs.
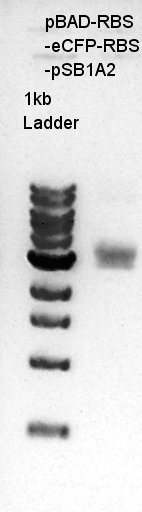
Colony PCR of pBAD-RBS-eCFP-RBS-pSB1A2
| DNA solution | 4 ul |
| Kapa-Taq(Taq polymerase) | 5 ul |
| Forward Primer(pbad-f2 primer) | 0.5 ul |
| Reverse Primer(PS-R down primer) | 0.5 ul |
| Total | 10 ul |
| Number | Degree | Second |
| 1 | 95 | 120 |
| 2 | 95 | 30 |
| 3 | 53.3 | 30 |
| 4 | 72 | 60 |
| 5 | 72 | 60 |
| 6 | 4 | HOLD |
Cycle:2~4 x 35
We used N1 (DW only) as controls. Desired product is about 900bp.
We thought that almost all of ligated DNA successfully ligated to our desired construct. We selected No. 1 colony for incubation and store No. 2,3 and 4 colony liquid medium at 4C.
Incubation of pBAD-RBS-eCFP-RBS-pSB1A2 for plasmid extraction
- Prepared 2 ml LBA into culture tubes.
- Re-suspended 1 colony mixture.
- Incubated at 37C for 18 hrs.
Transformation of ptet-RBS-eYFP-dT-pSTV28 and Test to confirm appropriate antibiotic amount for screening of pSTV28
To confirm how amount of chloramphenicol is needed for screening pSTV28 (Low copy plasmid) , we plated transformed cells on the special LBC which contains half amount of chloramphenicol we usually add. Now we call this LBC plate medium as LB1/2C.
- Mixed 2 ul ptet-RBS-eYFP-dT-pSTV28 ligation product to 50 ul of thawed competent cells on ice.
- Incubated on ice for 30 min.
- Mixed 350 ul of LB.
- Incubated for 2 hrs to get the resistance to Chloramphenicol.
- Prepared and Labeled two plastic plates with LB plate medium which contained appropriate antibiotics (LB1/2C).
- Plated 300 ul of the culture onto first dish and spread.
- Mixed 450 ul of LB to 50 ul of the culture and plated 300 ul of it onto second dish and spread.
- Incubated the plates at 37C for 19 hrs.
September 7th
Estimation of concentration of pBAD-RBS-eCFP-RBS-pSB1A2
We estimated the concentration of pBAD-RBS-eCFP-RBS-pSB1A2 is about 30 ng/ul.
Incubation of ptet-RBS-eYFP-dT-pSTV28 for plasmid extraction
- Prepared 5~6 ml LBC into culture tubes.
- Re-suspended 2 colony.
- Incubated at 37C for 7 hrs.
 "
"