Team:TU Darmstadt/Cooperation
From 2012.igem.org
(→Cooperation) |
(→Cooperation) |
||
| Line 3: | Line 3: | ||
For the Saccharomyces HMG CoA Reductase and Sulfolobus GGPP Synthase we were not able to build homology models, but their Saccharomyces FPP Synthase (SFPPS) worked out. The results for the developed models can be seen in the following pictures. <br><br> | For the Saccharomyces HMG CoA Reductase and Sulfolobus GGPP Synthase we were not able to build homology models, but their Saccharomyces FPP Synthase (SFPPS) worked out. The results for the developed models can be seen in the following pictures. <br><br> | ||
| - | <gallery perrow="2" widths="400px" heights=" | + | <gallery perrow="2" widths="400px" heights="250px" > |
File:FPP_Synthase_move_klein.gif|[[Animation of SFPPS]] | File:FPP_Synthase_move_klein.gif|[[Animation of SFPPS]] | ||
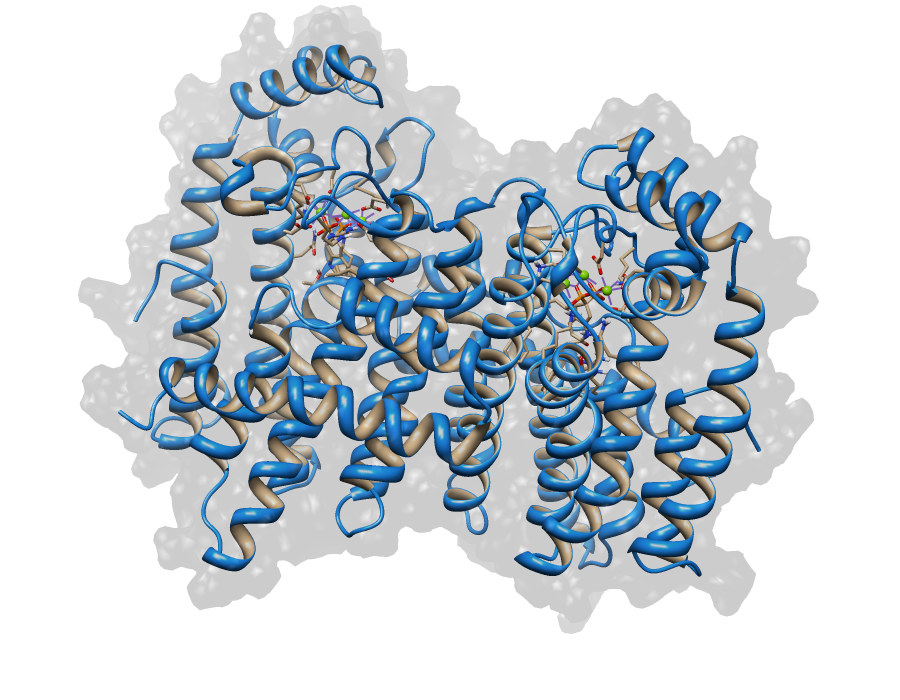
File:FPP_Synthase4.png|[[Top view of SFPPS]] | File:FPP_Synthase4.png|[[Top view of SFPPS]] | ||
| Line 15: | Line 15: | ||
For the target sequence of the Saccharomyces HMG CoA Reduktase (SHMGR) with 536 residues in 1 molecule. Hence the target sequence was the only available information we identified modelling templates by running various PSI-BLAST iterations. We used [http://www.rcsb.org/pdb/explore/explore.do?structureId=1DQA 1DQA] chain A (Human Hmg-coa Reductase) and [http://www.rcsb.org/pdb/explore/explore.do?structureId=2R4F 2R4F] chain A (3-hydroxy-3-methylglutaryl-coenzyme a Reductase). Unfortunately, our hybrid model could not be improved by copying parts from other models. <br> | For the target sequence of the Saccharomyces HMG CoA Reduktase (SHMGR) with 536 residues in 1 molecule. Hence the target sequence was the only available information we identified modelling templates by running various PSI-BLAST iterations. We used [http://www.rcsb.org/pdb/explore/explore.do?structureId=1DQA 1DQA] chain A (Human Hmg-coa Reductase) and [http://www.rcsb.org/pdb/explore/explore.do?structureId=2R4F 2R4F] chain A (3-hydroxy-3-methylglutaryl-coenzyme a Reductase). Unfortunately, our hybrid model could not be improved by copying parts from other models. <br> | ||
| - | <gallery perrow="2" widths="400px" heights=" | + | <gallery perrow="2" widths="400px" heights="250px" > |
File:Sulfolobus_GGPP_Synthase.png|[[Model of Sulfolobus GGPP Synthase (SGGPPS)]] | File:Sulfolobus_GGPP_Synthase.png|[[Model of Sulfolobus GGPP Synthase (SGGPPS)]] | ||
File:Saccharomyces_HMG_CoA_Reduktase.png|[[Aborted model of Saccharomyces HMG CoA Reduktase (SHMGR)]] | File:Saccharomyces_HMG_CoA_Reduktase.png|[[Aborted model of Saccharomyces HMG CoA Reduktase (SHMGR)]] | ||
</gallery> | </gallery> | ||
Revision as of 21:28, 26 September 2012
Cooperation
Capacity for teamwork is one major feature and character of the iGEM competition. Since we first met team frankfurt at the Achema our paths crossed on several occasions. During these occasions we came up with the idea to give them support by modelling their enzymes. The simulations group performed modelling studies for three enzymes of the Frankfurt iGEM Team.
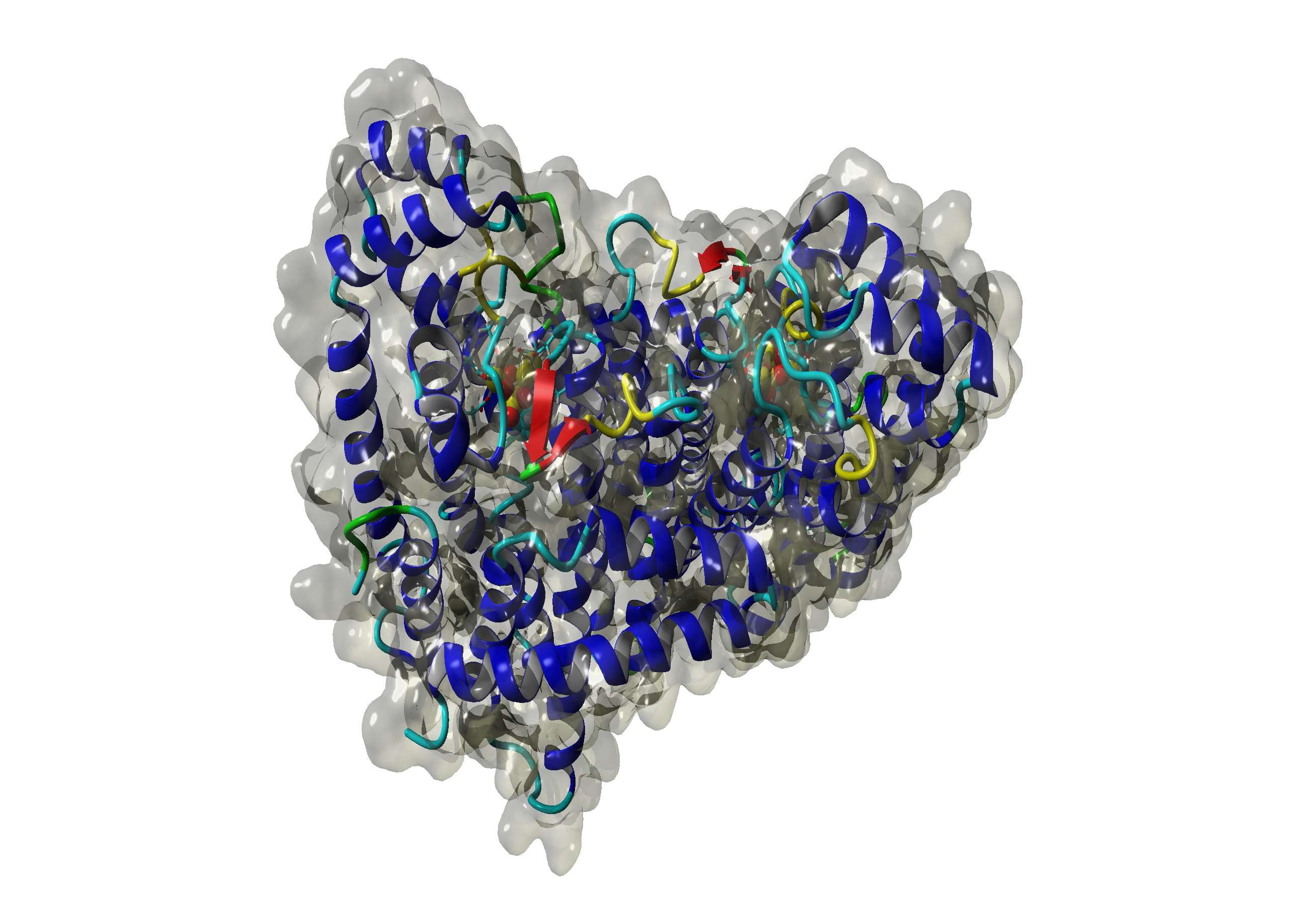
For the Saccharomyces HMG CoA Reductase and Sulfolobus GGPP Synthase we were not able to build homology models, but their Saccharomyces FPP Synthase (SFPPS) worked out. The results for the developed models can be seen in the following pictures.
FPP Synthase move klein.gif
|
|
The Sulfolobus GGPP Synthase (SGGPPS) sequence contains 262 residues in 1 molecule. We used [http://www.rcsb.org/pdb/explore/explore.do?structureId=1VIZ 1VIZ] chain A, [http://www.rcsb.org/pdb/explore/explore.do?structureId=2F6U 2F6U] chain B and [http://www.rcsb.org/pdb/explore/explore.do?structureId=3VK5 3VK5] chain A.
For the target sequence of the Saccharomyces HMG CoA Reduktase (SHMGR) with 536 residues in 1 molecule. Hence the target sequence was the only available information we identified modelling templates by running various PSI-BLAST iterations. We used [http://www.rcsb.org/pdb/explore/explore.do?structureId=1DQA 1DQA] chain A (Human Hmg-coa Reductase) and [http://www.rcsb.org/pdb/explore/explore.do?structureId=2R4F 2R4F] chain A (3-hydroxy-3-methylglutaryl-coenzyme a Reductase). Unfortunately, our hybrid model could not be improved by copying parts from other models.
 "
"