Team:ZJU-China/project.htm
From 2012.igem.org
(Difference between revisions)
| Line 398: | Line 398: | ||
<p>The Library may contain changes of self, self-assemble, RNA-RNA interaction, RNA-protein interaction. Some examples are show below.</p> | <p>The Library may contain changes of self, self-assemble, RNA-RNA interaction, RNA-protein interaction. Some examples are show below.</p> | ||
<p> </p> | <p> </p> | ||
| - | <p>1. Mutating arm length: changing the arm length of RNA scaffold D0. As the mechanism of D0 is reducing the distance of two key enzyme of the pathway, in other words, the output and reaction efficiency is depend on the local concentration. The two aptamer binding site in our project is on two hairpin arms witch are designed in the same length. The change of the arm length provides feasibility of distance-efficiency research. We used split GFP experiments. We made some mutations with different arm length, the result of D0M4 and D0M 5 split GFP experiment shows the light decreasing lend by split GFP FA-FB distance. The difference (PD0M4=0.079, PD0M5=0.025) suggests that the mutating arm length scaffold doesn’t provide an on/off switch but a definability one. It characterized the D0 in another way.</p> | + | <p>1.1 Mutating arm length: changing the arm length of RNA scaffold D0. As the mechanism of D0 is reducing the distance of two key enzyme of the pathway, in other words, the output and reaction efficiency is depend on the local concentration. The two aptamer binding site in our project is on two hairpin arms witch are designed in the same length. The change of the arm length provides feasibility of distance-efficiency research. We used split GFP experiments. We made some mutations with different arm length, the result of D0M4 and D0M 5 split GFP experiment shows the light decreasing lend by split GFP FA-FB distance. The difference (PD0M4=0.079, PD0M5=0.025) suggests that the mutating arm length scaffold doesn’t provide an on/off switch but a definability one. It characterized the D0 in another way.</p> |
<img src="https://static.igem.org/mediawiki/igem.org/d/da/Zju_library_Fig1a.jpg" width="500px" /> | <img src="https://static.igem.org/mediawiki/igem.org/d/da/Zju_library_Fig1a.jpg" width="500px" /> | ||
<p>fig 1a. D0 is the original scaffold. D0 a-d were mutated to the scaffold with different aptamer arm length. </p> | <p>fig 1a. D0 is the original scaffold. D0 a-d were mutated to the scaffold with different aptamer arm length. </p> | ||
| Line 404: | Line 404: | ||
<p>fig 1b. The result of arm length mutating. Both D0M4 and D0M5 scaffold half-on GEP.</p> | <p>fig 1b. The result of arm length mutating. Both D0M4 and D0M5 scaffold half-on GEP.</p> | ||
<p> </p> | <p> </p> | ||
| - | <p>1. | + | <p>1.2 Mutating aptamer binding site: Mutating the PP7 and MS2 binding sites prevented protein scaffolding. Preventing protein scaffolding lead to the key enzyme dissociation and the decrease of enzyme local concentration. By chancing the sequence of MS2 aptamer binding site, the fluorescent light decreased. D0M3 in our project is the molecular with mutated aptamer binding site. Split GFP experiment shows that there is a significant difference between D0 an D0M3(P≦0.05, fig2.c). Camille J. Delebecque has done the same work for the H2 biosynthesis pathway.</p> |
<img src="https://static.igem.org/mediawiki/igem.org/a/ad/Zju_library_Fig2a.jpg" width="500px" /> | <img src="https://static.igem.org/mediawiki/igem.org/a/ad/Zju_library_Fig2a.jpg" width="500px" /> | ||
<p>fig2a. MS2 and PP7 bind to the scaffold and make GFP work. </p> | <p>fig2a. MS2 and PP7 bind to the scaffold and make GFP work. </p> | ||
| Line 412: | Line 412: | ||
<p>fig2c. significant difference between D0 an D0M3</p> | <p>fig2c. significant difference between D0 an D0M3</p> | ||
<p> </p> | <p> </p> | ||
| - | <p>1. | + | <p>1.3 Assemblage: adding extra sequence for self-, RNA-, protein-assemblage. The added sequence may be a riboswitch, RNA or protein binding site, self-assemble structure. Regulation molecular search is also wanted synchronously. </p> |
<p> </p> | <p> </p> | ||
<p>Applications and outlook</p> | <p>Applications and outlook</p> | ||
<p> </p> | <p> </p> | ||
| - | <p> | + | <p>2.1 sRNA regulation: Simple an direct RNA-RNA interaction change the object RNA scaffold structure. As a Foundation regulation, it substantially enhances the possibilities of forthcoming experiment. </p> |
<img src="https://static.igem.org/mediawiki/igem.org/e/ec/Zju_library_Fig3.jpg" width="600px" /> | <img src="https://static.igem.org/mediawiki/igem.org/e/ec/Zju_library_Fig3.jpg" width="600px" /> | ||
| - | <p>fig3 The designed scaffold has a interaction to regulatory sRNA. Same mechanism, regulatory molecule can be changed to mRNA a. Turn off the scaffold by the competitive binding with aptamer binding site (green) b. The RNA scaffold has a secondary structural switch controls accessibility of sRNA-binding sites(blue) witch can change the arm length. Output regulated by arm length change. c. both methods were used. d. bind | + | <img src="https://static.igem.org/mediawiki/igem.org/8/89/Zju_library_Fig3d.jpg" width="600px" /> |
| + | <p>fig3 The designed scaffold has a interaction to regulatory sRNA. Same mechanism, regulatory molecule can be changed to mRNA a. Turn off the scaffold by the competitive binding with aptamer binding site (green) b. The RNA scaffold has a secondary structural switch controls accessibility of sRNA-binding sites(blue) witch can change the arm length. Output regulated by arm length change. c. both methods were used. d. bind and release the object molecular.)</p> | ||
<p> </p> | <p> </p> | ||
| - | <p> | + | <p>2.2 Protein expression (mRNA) regulation: RNA scaffold as a free molecular in cell can specific bind mRNA and protein. Binding molecular changes the structure of scaffold to release or combine something. So that oncogene and virogene can be found and controlled by the drug from RNA scaffold. The problem of cancer therapeutic drug side effecting may solved by it. </p> |
<p> </p> | <p> </p> | ||
| - | <p> | + | <p>2.3 Self quenching(Self regulation): Adding self binding site, a balance of “on” and “off” scaffolds is built. The relationship between the binding site size, CG bases, binding form and the rate binding molecular is urgently modeled. Forming dimerization and trimerization, the concentration of working scaffold could be regulated.</p> \ |
<p> </p> | <p> </p> | ||
| - | <p> | + | <p>2.4 Polo-scaffold: Scaffold with intermolecular binding component. These scaffolds bind each other or bind through mediate molecular. And this binding mode has been proved both in vitro and vivo. The aggregation of molecular also makes artificial organelle achievable. </p> |
<img src="https://static.igem.org/mediawiki/igem.org/2/2f/Zju_library_Fig4a.jpg" width="600px" /> | <img src="https://static.igem.org/mediawiki/igem.org/2/2f/Zju_library_Fig4a.jpg" width="600px" /> | ||
| Line 433: | Line 434: | ||
<p>fig4 c. Polo-scaffold be made by head-tail binding and.</p> | <p>fig4 c. Polo-scaffold be made by head-tail binding and.</p> | ||
<p> </p> | <p> </p> | ||
| - | |||
| - | |||
<p> </p> | <p> </p> | ||
<p>Several RNA scaffold mutations are constructed and characterize, but they are the tip of the iceberg. There is still plenty to do in this part. The charms of library are the selection and combination. It introduces a new concept of biobrick combination mode.</p> | <p>Several RNA scaffold mutations are constructed and characterize, but they are the tip of the iceberg. There is still plenty to do in this part. The charms of library are the selection and combination. It introduces a new concept of biobrick combination mode.</p> | ||
| Line 446: | Line 445: | ||
<!--Content Goes Here--> | <!--Content Goes Here--> | ||
| - | <div | + | <div style="height:800px;overflow:scroll;"> |
| - | + | <p align="justify"> </p> | |
| - | + | <p align="justify">In previous work, FA and FB are used to indicate the efficiency of riboscaffold. In order to further prove the function of riboscaffold, we plan to substitute FA, FB with functional enzymes or protein substrates like ferredoxin in hydrogen producing pathway respectively. </p> | |
| - | + | <p align="justify"> </p> | |
| - | + | <p align="justify">Considering the availability of material and abundant parts distributed by iGEM, we search the 2012 kit plate1-5 to find optimal pathways. After a pre-selection, six pathways are on candidate list. For sake of experimental feasibility, we perform a further selection based on several caritas as follows:</p> | |
| - | + | <p align="justify"> </p> | |
| - | + | <p align="justify">1. Product is easy to detect and measure;</p> | |
| - | + | <p align="justify"> </p> | |
| - | + | <p align="justify">2. Substrate is easy to get;</p> | |
| - | + | <p align="justify"> </p> | |
| - | + | <p align="justify">3. Product is beneficial to human;</p> | |
| - | + | <p align="justify"> </p> | |
| - | + | <p align="justify">4. The length of amino acid sequence of enzyme is optimal to be fusion protein;</p> | |
| - | + | <p align="justify"> </p> | |
| - | + | <p align="justify">5. Two proteins involved in the basic pathway.</p> | |
| - | + | <p align="justify"> </p> | |
| - | + | <p align="justify">Candidate list:</p> | |
| - | + | <p align="justify"> </p> | |
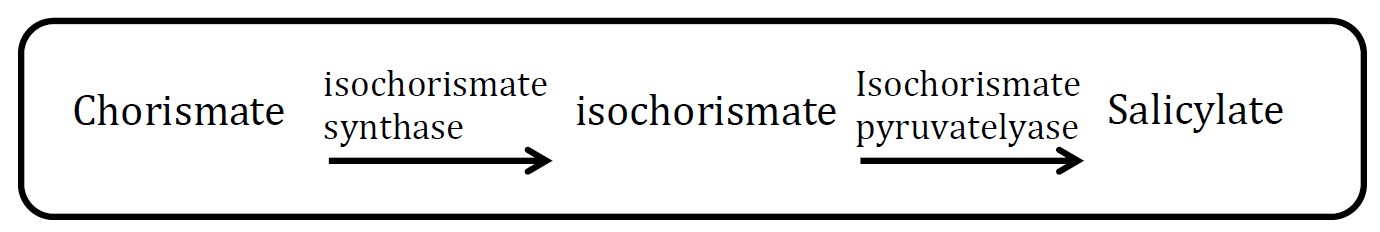
| - | + | <h2>1. Salicylate pathway</h2> | |
| - | + | <p>(Group: iGEM2006_MIT)</p> | |
| - | + | <img src="http://www.jiajunlu.com/igem/zju_iaa_1.jpg" width="600px" /> | |
| - | + | <p align="justify"> </p> | |
| - | + | <p align="justify">Assessment: </p> | |
| - | + | <p align="justify"> </p> | |
| - | + | <p align="justify">The characterization method of gas chromatography is difficult to perform. First, what can be analyzed is methyl salicylate production, that is to say, another enzyme should be co-transformed to E.coli too, which will increase cell’s burden and reduce the ratio of successful co-transformation. Second, it is not convenient for us to borrow the relative machine.</p> | |
| + | <p align="justify"> </p> | ||
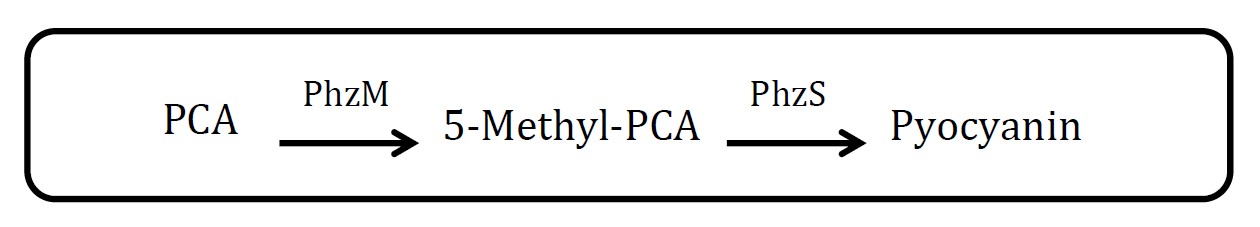
| + | <h2>2. Pyocyanin pathway</h2> | ||
| + | <p>(Group: iGEM2007_Glasgow)</p> | ||
| + | <img src="http://www.jiajunlu.com/igem/zju_iaa_2.jpg" width="600px" /> | ||
| + | <p align="justify"> </p> | ||
| + | <p align="justify">Assessment: </p> | ||
| + | <p align="justify"> </p> | ||
| + | <p align="justify">Through there are exactly two enzymes involved in this pathway, but the source of material, phenazine-1-carboxylic acid (PCA), is not mentioned. And it not easy to measure the amount of pyocyanin. </p> | ||
| + | <p align="justify"> </p> | ||
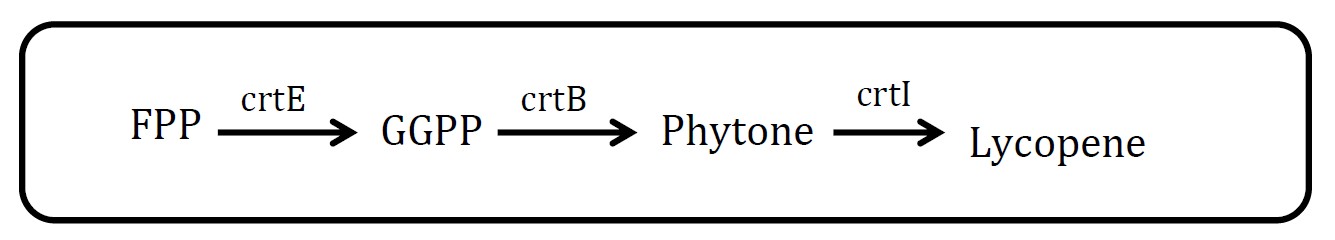
| + | <h2>3. Lycopene pathway</h2> | ||
| + | <p>(Group: iGEM2009_Cambridge) </p> | ||
| + | <img src="http://www.jiajunlu.com/igem/zju_iaa_3.jpg" width="600px" /> | ||
| + | <p align="justify"> </p> | ||
| + | <p align="justify">Assessment: </p> | ||
| + | <p align="justify"> </p> | ||
| + | <p align="justify">Lycopene is visible red and its substrate, FPP, is colorless. So measurement is quite feasible. But there are at least three proteins in this pathway, which will increase the burden of cell. But in future work, we could have a try.</p> | ||
| + | <p align="justify"> </p> | ||
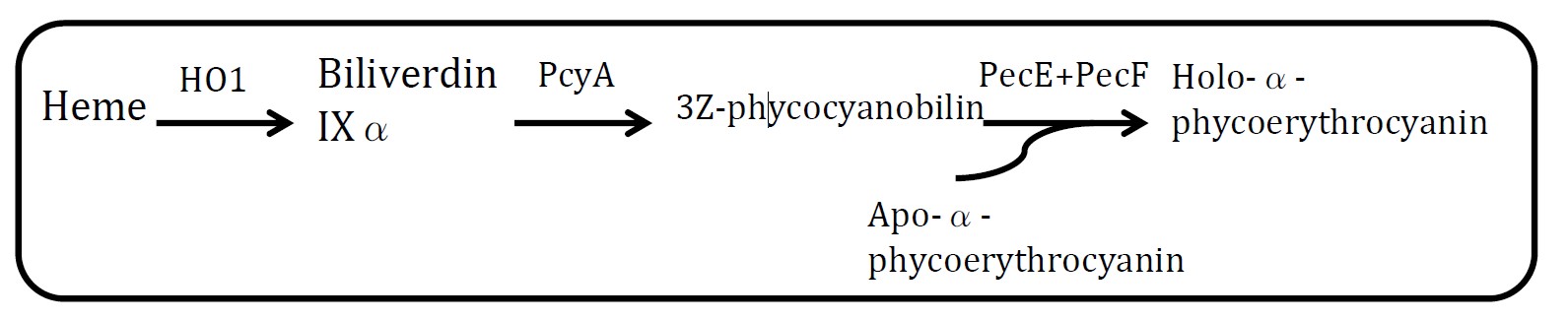
| + | <h2>4. Holo-α-phycoerythrocyanin pathway</h2> | ||
| + | <p>(Group: iGEM2004_UTAustin)</p> | ||
| + | <img src="http://www.jiajunlu.com/igem/zju_iaa_4.jpg" width="600px" /> | ||
| + | <p align="justify"> </p> | ||
| + | <p align="justify">Assessment: </p> | ||
| + | <p align="justify"> </p> | ||
| + | <p align="justify">Heme is metabolic product of E.coli and Holo-α-phycoerythrocyanin is blue. But at least 5 proteins should be expressed in E.coli.</p> | ||
| + | <p align="justify"> </p> | ||
| + | <h2>5. BPA degradation pathway</h2> | ||
| + | <p>(Group: iGEM2008_University_of_Alberta)</p> | ||
| + | <p align="justify"> </p> | ||
| + | <p align="justify">Assessment: </p> | ||
| + | <p align="justify"> </p> | ||
| + | <p align="justify">Bisphenol A is degraded by BisdA and BisdB. But BPA is toxic to cells.</p> | ||
| + | <p align="justify"> </p> | ||
| + | <h2>6. IAM pathway</h2> | ||
| + | <p>(Group: iGEM2011_Imperial)</p> | ||
| + | <img src="https://static.igem.org/mediawiki/2012/c/c3/ZJU_IAA_Screen_Shot_2012-09-27.png" width="600px" /> | ||
| + | <p align="justify"> </p> | ||
| + | <p align="justify">Assessment: </p> | ||
| + | <p align="justify"> </p> | ||
| + | <p align="justify">Five pathways described above all have some drawbacks, finally, only one pathway left, IAM pathway. The two-step IAM pathway generates indole-3-acetic acid (IAA) from the precursor tryptophan. IAA tryptophan monooxygenase (IaaM) catalyses the oxidative carboxylation of L-tryptophan to indole-3-acetamide, which is hydrolysed to IAA and ammonia by indoleacetamide hydrolase (IaaH). </p> | ||
| + | <p align="justify"> </p> | ||
| + | <p align="justify">Final Decision: </p> | ||
| + | <p align="justify"> </p> | ||
| + | <img src="https://static.igem.org/mediawiki/2012/4/4f/ZJU_S3_candidate01.png" width="600px" /> | ||
| + | </div> | ||
</div><!-- end .acc_container --> | </div><!-- end .acc_container --> | ||
Revision as of 20:24, 26 September 2012

 "
"