Team:Cornell/Notebook/Nah operon
From 2012.igem.org
(Difference between revisions)
(→June 22nd, Friday) |
(→June 22nd, Friday) |
||
| Line 34: | Line 34: | ||
:[https://2011.igem.org/Team:Cornell/Protocols Miniprepped] directly transformed Gibson Assembly product for sequencing using the the Gibson nah1F and nah4R primers (each w/ 20 bp overhangs). | :[https://2011.igem.org/Team:Cornell/Protocols Miniprepped] directly transformed Gibson Assembly product for sequencing using the the Gibson nah1F and nah4R primers (each w/ 20 bp overhangs). | ||
:Ran undigested miniprep with [https://2011.igem.org/Team:Cornell/Protocols gel electrophoresis], looking for large bands corresponding to supercoiled DNA. The gel shows distinct bands for all three lanes. We interpreted this to mean that we got product. Submitted for [https://2011.igem.org/Team:Cornell/Protocols sequencing] | :Ran undigested miniprep with [https://2011.igem.org/Team:Cornell/Protocols gel electrophoresis], looking for large bands corresponding to supercoiled DNA. The gel shows distinct bands for all three lanes. We interpreted this to mean that we got product. Submitted for [https://2011.igem.org/Team:Cornell/Protocols sequencing] | ||
| - | [[File:Cornell2012_0621_Gibson-entire-plasmid.jpg|600px]] | + | ::[[File:Cornell2012_0621_Gibson-entire-plasmid.jpg|600px]] |
===June 24th-30th=== | ===June 24th-30th=== | ||
Revision as of 21:07, 28 June 2012
| Home | Team | Project | Parts | Modeling | Notebook | Protocols | Safety | Attributions |
|---|
Contents |
June
Project Description
The purpose of this subproject is to make the nah operon biobrick compatible for the use of future iGEM teams. The nah operon is an operon that converts napthalene into salicylate. In our system, this will allow napthalene to be detected by the salicylate reporter. There are three standard cutsites internal to the operon which we are removing - the biobrick compatible piece could be used by future teams in bioremediation projects.
June 10th-16th
June 13th, Wednesday
- Set up PCR for four fragments of the nah operon as well as the pBMT-1 backbone, using primers designed to mutate cut-sites concurrently with Gibson Assembly. Also set up a PCR for the entire nah operon. Due to length of the fragments, a longer extension time was chosen.
- If Gibson Assembly fails, but we are able to PCR the entire 10kb nah operon using Phusion polymerase, an alternate method of mutation using Phusion will be pursued.
- Work done by: Caleb, Claire, Dylan, Spencer, Steven, and Swati
June 14th, Thursday
- PCR only amplified nah1, nah3, and nah4 - longer fragments (nah2, the full nah operon, and pBMT-1) were not identified on the PCR product gel. Set up another PCR with shorter extension time.
June 15th, Friday
- PCR of four out of five products visible on gel. Set up PCR of pBMT-1, final fragment required for Gibson Assembly of the nah operon.
June 17th-23rd
June 17th, Sunday
Successful PCR of pBMT-1 gel purified.
June 19th, Tuesday
- Ran Gibson Assembly of nah operon fragments into pBMT-1 backbone and transformed into DH5a electrocompotent E. coli cells.
- Set up PCR to amplify the Gibson Assembly products.
- Work done by: Dylan and Swati
June 20th, Wednesday
- Set up a digestion of the PCR of Gibson Assembly products and ran the digested products on a gel to see if Gibson worked.
- Three colonies on plates of DH5a transformed with Gibson Assembly product. Made liquid cultures to miniprep and sequence.
- Work done by: Dylan and Swati
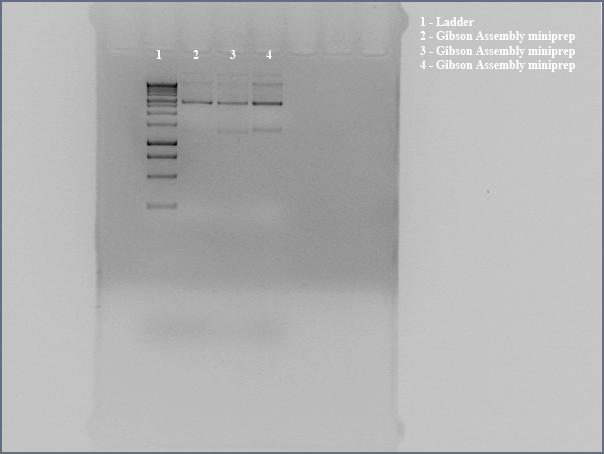
June 22nd, Friday
- Miniprepped directly transformed Gibson Assembly product for sequencing using the the Gibson nah1F and nah4R primers (each w/ 20 bp overhangs).
- Ran undigested miniprep with gel electrophoresis, looking for large bands corresponding to supercoiled DNA. The gel shows distinct bands for all three lanes. We interpreted this to mean that we got product. Submitted for sequencing
June 24th-30th
June 27th, Wednesday
- Ran digest of gibson-assembled nah operon with gel electrophoresis
====June 28th, Thursday====
 "
"