|
Sensory BioBrick systems have been a large constituent of previous iGEM projects in which teams have combined impressive amounts of logic with limitless creativity in order to produce synthetically engineered organisms with the ability to detect the presence of specific substrates; this was achieved by combining various promoters and reporters to produce novel gene systems of great breadth and depth.
We too have taken a sensory approach to our project and have produced systems involved in the sensation of nitric oxide (NO). Originally we set out to develop a bacterial and mammalian hybrid NO-sensing promoter (which we have achieved); we then looked into ways of quantifying the levels of highly reactive and difficult to measure NO within a system, leading to us producing a novel gene regulation system known as the comparator circuit. Throughout the project we went on to look at theoretical alternative approaches to the gene systems we have produced.
Overall in our project we have produced 6 sensory BioBricks, 2 BioBricks involved in gene regulation, and have further characterised 4 more BioBricks. All 8 of our BioBricks have been submitted to the registry and the 6 sensory BioBricks have been characterised.
Our hybrid promoter hopes to add to the systems already in the registry by creating a hybrid promoter that combines the bacterial promoter PyeaR and the mammalian CArG element , both of which respond to exogenous nitrogenous species. Combining the two would allow a more modular nitric oxide sensor that can be used in mammalian and bacterial cells interchangeably.
Six new biobricks produced and submitted to the registry with characterisation from fluorescence-based experiments.
Parts produced from this project:
[http://partsregistry.org/wiki/index.php?title=Part:BBa_K774000 Bacterial-Mammalian/B-M (PyeaR-CArG) Hybrid Promoter] -- [http://partsregistry.org/wiki/index.php?title=Part:BBa_K774001 Mammalian-Bacterial/M-B (CArG-PyeaR) Hybrid Promoter] -- [http://partsregistry.org/wiki/index.php?title=Part:BBa_K774004 B-M + eCFP] -- [http://partsregistry.org/wiki/index.php?title=Part:BBa_K774005 B-M + RFP] -- [http://partsregistry.org/wiki/index.php?title=Part:BBa_K774006 M-B + eCFP] -- [http://partsregistry.org/wiki/index.php?title=Part:BBa_K774004 M-B + RFP]
Parts characterised from this project:
[http://partsregistry.org/wiki/index.php?title=Part:BBa_K216005 PyeaR Promoter] -- [http://partsregistry.org/Part:BBa_K381001 PyeaR Promoter + GFP] -- [http://partsregistry.org/Part:BBa_E0420 enhanced Cyan Fluorescent Protein + RBS + Terminators] -- [http://partsregistry.org/Part:BBa_K081014 Red Fluorescent Protein + RBS + Terminators]
Our main project has resulted in the production of a hybrid bacterial and mammalian promoter optimised for induction by nitric oxide, nitrates and nitrites. We have ligated PyeaR, a known bacterial promoter and [http://partsregistry.org/wiki/index.php?title=Part:BBa_K216005 Part BBa_K216005] (Cambridge 2009) in the parts registry, with its mammalian counterpart, CArG. The resulting hybrid promoter has been synthesised in two orientations; PyeaR (bacterial, B) upstream of CArG (mammalian, M), nicknamed (B-M); and CArG upstream of PyeaR (M-B). These orientations were submitted to the parts registry as our first two biobricks.
Each orientation of the promoter was ligated to enhanced cyan fluorescent protein (eCFP) and red fluorescent protein (RFP) to produce four new biobricks which have been submitted to the parts registry. These promoter + fluorescent protein biobricks have been characterised following transformation into Escherichia coli and induction by potassium nitrate using methods such as flow cytometry, fluorescence-activated cell sorting (FACS) and scanning with a fluorometer. The data from these experiments has proved that our promoter works as we expected it to. We have also transfected M-B + eCFP into a human breast cancer cell line, MCF7, and have proved the system is flexible and can be used in both eukaryotes and prokaryotes.
We believe the promoters we have produced have relevant uses in cancer therapeutics, soil fertilisation and detection of emissions from industries such as construction.
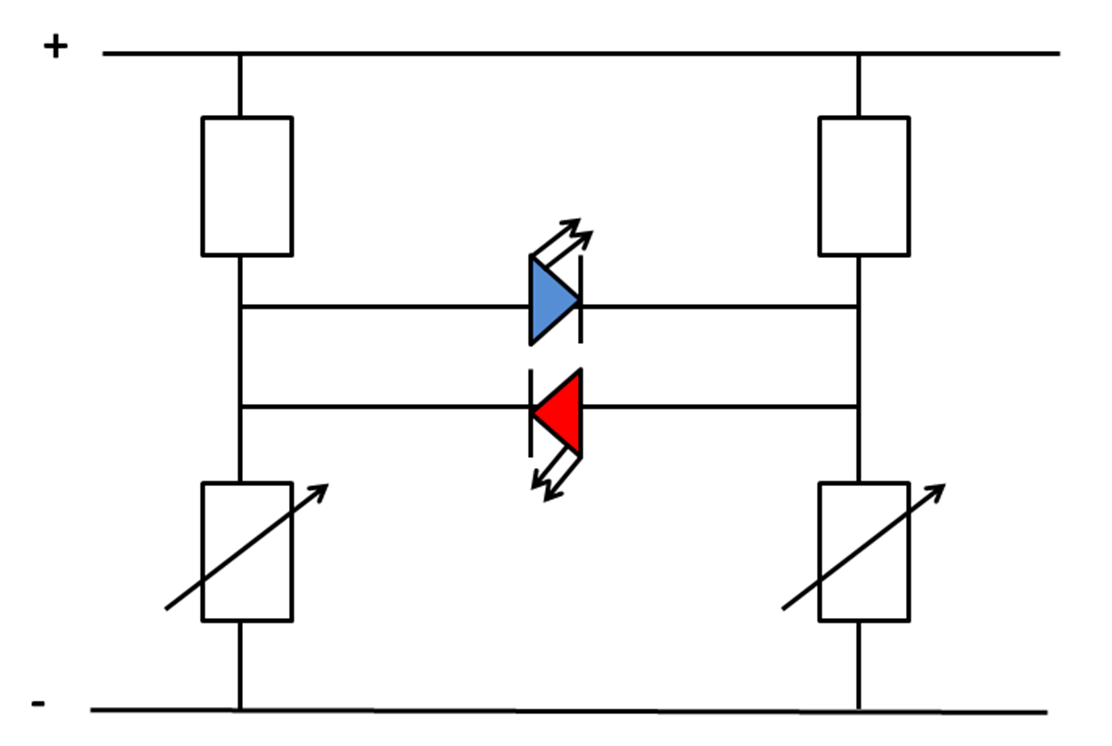 Figure 3. The electrical circuitary of the comparator circuit. The lack of specificity of the bacterial promoter, pYEAR, used in the hybrid promoter was a pitfall that was always a concern. From this potential problem spawned a potential solution; the Comparator Circuit.This pair of BioBricks is designed to specifically bind to each other while ligated to two different promoters of overlapping specificity to result in an integrating of the conflicting outputs of the two opposing gene systems.
Our system relies on two constructs that interact via complimentary base pair sequences both before and after the ribosome binding site of the reporter protein. The idea being that, when both transcripts are present in the chassis, they would bind together, inhibiting the translation of the reporter proteins.
Any imbalance of transcription due to the presence of the substrate of interest results in free mRNA of the gene system that detects that substrate. Crucially, if both promoters detect the same substrates but differ with one extra substrate being detected by one of the promoters, it is this substrate and this substrate only that our system will be able to detect in a simple and quantitative way.
Our team have constructed a countercurrent comparator circuit in which the reporter proteins are at the same end of the complimentary region, although a contracurrent system has been theorised. Both systems share a crucial subtractive nature comparable to an analogue computer. We envisage that, should the system be fine-tuned and expanded on, a variety of different business sectors from agriculture to spinoff pharmaceutical companies (Please check out our Quanticare page for more details) could capitalise on this novel genetic technology.
What we have produced is a biobrick pair that work in harmony, when ligated to promoters of interest and genes of interest, to sequester translation when both mRNA transcripts are present in the cell. The use of quantative tuners with these biobricks is encouraged to ensure that the transcription rate both gene constructs are equal when both promoters are transcribing at their optimal rate. Although the parts have been submitted to the registry and theoretically characterised, time constraints have meant that further lab-based characterisation could not occur.
However, we hope to utilise any free time in our timetables sduring the next semester to characterise the biobricks further (please see our project proposal), and hope that we will be given a chance to present our further findings at MIT!
To conclude, what we have created is a pair of antagonistic BioBricks that turned the pair of mRNAs in which they reside into translational repressor molecules when both are transcribed in tandum within a specific chassis of interest, a new application for mRNA complimentary base pairing within the registry and a project we feel could go very far indeed.
Division circuit
The comparator circuit that we have created integrates two different transcription levels in a negative manner (subtraction) to perform a range of functions a range of different integrations (calculations) are necessary to this end we have also designed a system that would allow one signal (transcription rate) to be divided by the transcription level of another promoter. To achieve this we have used the processes of attenuation and the three loop system (in the tryp leader). the system has also been mathematically modelled. to find more please click the image above.
Multisensor
There are many groups of chemical species for which there are no current biological techniques for distinguishing between each of these species and quantitatively analysing its concentration. Here we outline a possible approach for solving this problem using non-specific transcription factors and promoters. We use nitrates nitrites and nitric oxide as our example group. To find more please click the image above.
in addition to the two main projects, we have also worked to elaborate on some of the teams earlier ideas during the project.
|
 "
"