Team:Colombia/Project/Experiments/Pseudomonas
From 2012.igem.org
Sylvita1015 (Talk | contribs) |
Sylvita1015 (Talk | contribs) |
||
| Line 23: | Line 23: | ||
[[File:Confirmacion TA Cloramfenicol.png|center|450px]] | [[File:Confirmacion TA Cloramfenicol.png|center|450px]] | ||
| - | We were going to use the | + | We were going to use the ''wild type'' promoters of the Toxin-Antitoxin modules; however the deterministic model gave us feedback which established that inducible promoters were much more likely to give us the results we were looking for. |
| + | |||
| + | For the construction of our inducible parts, we first tried to build the toxins under the control of the Lac promoter and the antitoxins under the tetR promoter. We succeeded in the construction of the parts containing the HipB and ''istR'' antitoxins and the MqsR toxin. | ||
| + | |||
| + | But due to the leakage of lac promoter and even that of the tetR promoter (has less lekeage that lac) we were unable to construct the remaining toxin parts using both of those promoters. To overcome this inconvenience we decided to use the pmr promoter which is inducible by the CI lambda phage protein. So far we have constructed the inducible HipA7 part with the aforementioned promoter. | ||
| + | |||
In order to assess the functionality of these parts, we cloned HipB under the lac promoter in an E. coli strain that contains hipA7 in its chromosome (E. coli k12mg1655 th1269). As HipB neutralizes the induction of persistency caused by HipA7 in this strain, we measured the persisters frequency after induction with different concentrations of IPTG and compared it with the normal levels of persistency in this strain. | In order to assess the functionality of these parts, we cloned HipB under the lac promoter in an E. coli strain that contains hipA7 in its chromosome (E. coli k12mg1655 th1269). As HipB neutralizes the induction of persistency caused by HipA7 in this strain, we measured the persisters frequency after induction with different concentrations of IPTG and compared it with the normal levels of persistency in this strain. | ||
We are in the process of making inducible CI under the control of the lac promoter in order to analyze the effect of the induction of HipA7 in the persisters frequency on a wild type E. coli strain. | We are in the process of making inducible CI under the control of the lac promoter in order to analyze the effect of the induction of HipA7 in the persisters frequency on a wild type E. coli strain. | ||
Revision as of 22:12, 25 September 2012
Template:Https://2012.igem.org/User:Tabima
Whoops! Site in construction. Please come back later for future updates!
Toxin-Antitoxin
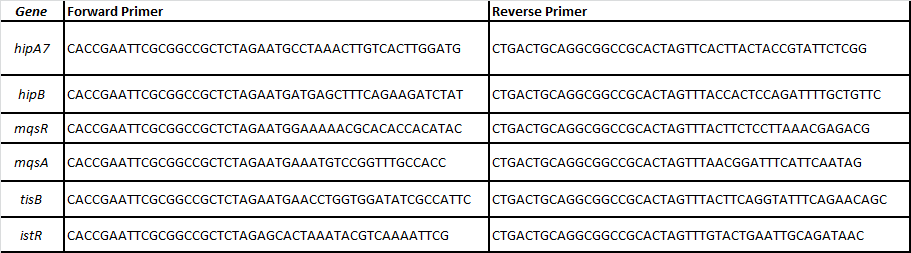
We designed specific primers for each ORF of the following toxin-antitoxin pairs related with persistence (Balaban et al, 2004; Lewis 2010; Maisonneuve et al, 2011):
-HipA7/HipB
-MqsR/MqsA
-TisB/istR
In order to enable other teams to use said toxin-antitoxin modules we cloned these parts without promoter in chloramphenicol backbones.
We were going to use the wild type promoters of the Toxin-Antitoxin modules; however the deterministic model gave us feedback which established that inducible promoters were much more likely to give us the results we were looking for.
For the construction of our inducible parts, we first tried to build the toxins under the control of the Lac promoter and the antitoxins under the tetR promoter. We succeeded in the construction of the parts containing the HipB and istR antitoxins and the MqsR toxin.
But due to the leakage of lac promoter and even that of the tetR promoter (has less lekeage that lac) we were unable to construct the remaining toxin parts using both of those promoters. To overcome this inconvenience we decided to use the pmr promoter which is inducible by the CI lambda phage protein. So far we have constructed the inducible HipA7 part with the aforementioned promoter.
In order to assess the functionality of these parts, we cloned HipB under the lac promoter in an E. coli strain that contains hipA7 in its chromosome (E. coli k12mg1655 th1269). As HipB neutralizes the induction of persistency caused by HipA7 in this strain, we measured the persisters frequency after induction with different concentrations of IPTG and compared it with the normal levels of persistency in this strain. We are in the process of making inducible CI under the control of the lac promoter in order to analyze the effect of the induction of HipA7 in the persisters frequency on a wild type E. coli strain.
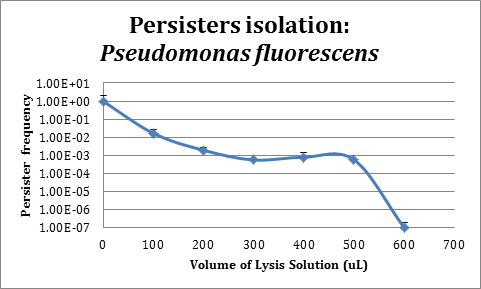
Assays in Pseudomonas fluorescens
In order to improve the stability and survival of our system we are assessing the possibility of using P. fluorescens as our final chassis. For this purpose, we first standardized the normal recount of persisters without induction. We used a protocol based on lysis (manuscript in preparation) for persisters isolation.
 "
"