Team:Chalmers-Gothenburg/Results
From 2012.igem.org
(→Expression of the gene for the LH/CG receptor in yeast) |
m (→Deletion of CWP2 gene) |
||
| Line 73: | Line 73: | ||
In order to test if the cells remained alive in the urin, 300 µl of each culture (after 4h) were taken and OD<SUB>600</SUB> was adjusted to 0.5. The samples were diluted 3x, 9x, 27x and 81x and 10 µl aliquots of each dilution were spotted on an YPD plate (Figure 2). The cells from both the YPD and the urine media could grow, this means that the cells survived 4 h in urine and grew normal after being spotted on a YPD plate. Summarizing, yeast cells are unable to proliferate in the urine medium but do survive under these conditions. | In order to test if the cells remained alive in the urin, 300 µl of each culture (after 4h) were taken and OD<SUB>600</SUB> was adjusted to 0.5. The samples were diluted 3x, 9x, 27x and 81x and 10 µl aliquots of each dilution were spotted on an YPD plate (Figure 2). The cells from both the YPD and the urine media could grow, this means that the cells survived 4 h in urine and grew normal after being spotted on a YPD plate. Summarizing, yeast cells are unable to proliferate in the urine medium but do survive under these conditions. | ||
| - | + | ='''Deletion of ''CWP2'' gene'''= | |
| - | + | ||
One task of our iGEM project was the deletion of the ''CWP2'' gene, which is encoding a cell wall mannoprotein. By removing it, we aimed for higher cell wall permeability and thus enhanced chances of our ligand hCG to pass the cell wall and to bind to the membrane-bound receptor. | One task of our iGEM project was the deletion of the ''CWP2'' gene, which is encoding a cell wall mannoprotein. By removing it, we aimed for higher cell wall permeability and thus enhanced chances of our ligand hCG to pass the cell wall and to bind to the membrane-bound receptor. | ||
| + | |||
| + | ==Gene Deletion according to the Bipartite method== | ||
The gene deletion was performed according to the bipartite method. The results from the first PCR reactions, in which we amplified the overlapping fragments, can be seen in Figure 3. | The gene deletion was performed according to the bipartite method. The results from the first PCR reactions, in which we amplified the overlapping fragments, can be seen in Figure 3. | ||
[[File:Resultpcr1.jpg|thumb|600px|center|Figure 3: A) A schematic illustration of the first four PCR reactions. The 5' and 3' end of the kanMX (kanamycin resistance) cassette, flanked to loxP sites and available in vector pUG6h, were amplified by PCR (PCR reaction 1 and 2). In addition, 500 bp of the up- and downstream sequence of the ''CWP2'' gene were amplified from genomic DNA using primers with 5' extensions that are complementary to the loxP sites as indicated with lines above. B) The results of the four PCR reactions on gel. The expected sizes of the kanMX and ''CWP2'' fragments are about 1000 bp and 500 bp respectively which correspond to the sizes observed on gel.]] | [[File:Resultpcr1.jpg|thumb|600px|center|Figure 3: A) A schematic illustration of the first four PCR reactions. The 5' and 3' end of the kanMX (kanamycin resistance) cassette, flanked to loxP sites and available in vector pUG6h, were amplified by PCR (PCR reaction 1 and 2). In addition, 500 bp of the up- and downstream sequence of the ''CWP2'' gene were amplified from genomic DNA using primers with 5' extensions that are complementary to the loxP sites as indicated with lines above. B) The results of the four PCR reactions on gel. The expected sizes of the kanMX and ''CWP2'' fragments are about 1000 bp and 500 bp respectively which correspond to the sizes observed on gel.]] | ||
| Line 83: | Line 84: | ||
[[File:Pcr2result.jpg|thumb|600px|center|Figure 4: A) A schematic illustration of the fusion PCR, the second step in the bipartite method. The ''CWP2'' upstream fragment was fused with the 5’ end of the kanMX cassette and the ''CWP2'' downstream fragment was fused with the 3’ end of the kanMX cassette. B) The results of the fusion PCR on gel. Two samples (A and B) of each PCR reaction are shown. The expected size of both fusion fragments is about 1500 bp which corresponds to the sizes observed on gel (indicated with an arrow).]] | [[File:Pcr2result.jpg|thumb|600px|center|Figure 4: A) A schematic illustration of the fusion PCR, the second step in the bipartite method. The ''CWP2'' upstream fragment was fused with the 5’ end of the kanMX cassette and the ''CWP2'' downstream fragment was fused with the 3’ end of the kanMX cassette. B) The results of the fusion PCR on gel. Two samples (A and B) of each PCR reaction are shown. The expected size of both fusion fragments is about 1500 bp which corresponds to the sizes observed on gel (indicated with an arrow).]] | ||
| - | + | ==Colony PCR== | |
Yeast strain IMFD-73 was transformed with 150 ng of each fragment and grown on G418 plates. Colonies could be observed, which were purified, and colony PCR was then performed in order to test whether the kanMX cassette was integrated randomly in the genome or had replaced the ''CWP2'' gene as desired. PCR was run with a forward primer outside of the inserted region and reverse primers within the ''CWP2'' gene or kanMX cassette respectively. As a control, a wild-type strain and a Δ''cwp2'' strain from a deletion library were also tested. The results of the PCR reactions indicated that the deletion was correct (see Figure 5). | Yeast strain IMFD-73 was transformed with 150 ng of each fragment and grown on G418 plates. Colonies could be observed, which were purified, and colony PCR was then performed in order to test whether the kanMX cassette was integrated randomly in the genome or had replaced the ''CWP2'' gene as desired. PCR was run with a forward primer outside of the inserted region and reverse primers within the ''CWP2'' gene or kanMX cassette respectively. As a control, a wild-type strain and a Δ''cwp2'' strain from a deletion library were also tested. The results of the PCR reactions indicated that the deletion was correct (see Figure 5). | ||
[[File:Testpcr_total.jpg|thumb|600px|center|Figure 5: A) Schematic illustration of the colony PCR. A forward primer was designed to bind upstream in the genomic DNA of the 500 bp region that had been used in the construction of the bipartite fragment. Two reverse primers were designed to hybridize within the ''CWP2'' gene and in the kanMX cassette respectively. The primers are indicated with arrows. B) The results of the PCR reactions on gel. In every reaction, the cwp2-fw primer but different reverse primers were used. 1: The Δ''cwp2'' deletion strain (bipartite) with the kanMX-rev primer. 2: The Δ''cwp2'' deletion strain (from deletion library) with the kanMX-rev primer. 3: The Δ''cwp2'' deletion strain (bipartite) with the cwp2-rev primer. 4: The Δ''cwp2'' deletion strain (from deletion library) with the cwp2-rev primer. 5. A wild type yeast strain with the cwp2-rev primer. Since the ''CWP2'' gene is missing and the kanMX cassette is inserted instead, bands are expected when PCR is run with the kanMX-rev primer (1, 2, expected size 1800 bp) but no bands should be observed when PCR is performed with the cwp2-rev primer (3, 4). On the contrary, a clear band of about 800 bp should be observed when running PCR with the wild type strain and the cwp2-rev primer. This corresponds to the result on gel which indicates that the ''CWP2'' deletion was successful.]] | [[File:Testpcr_total.jpg|thumb|600px|center|Figure 5: A) Schematic illustration of the colony PCR. A forward primer was designed to bind upstream in the genomic DNA of the 500 bp region that had been used in the construction of the bipartite fragment. Two reverse primers were designed to hybridize within the ''CWP2'' gene and in the kanMX cassette respectively. The primers are indicated with arrows. B) The results of the PCR reactions on gel. In every reaction, the cwp2-fw primer but different reverse primers were used. 1: The Δ''cwp2'' deletion strain (bipartite) with the kanMX-rev primer. 2: The Δ''cwp2'' deletion strain (from deletion library) with the kanMX-rev primer. 3: The Δ''cwp2'' deletion strain (bipartite) with the cwp2-rev primer. 4: The Δ''cwp2'' deletion strain (from deletion library) with the cwp2-rev primer. 5. A wild type yeast strain with the cwp2-rev primer. Since the ''CWP2'' gene is missing and the kanMX cassette is inserted instead, bands are expected when PCR is run with the kanMX-rev primer (1, 2, expected size 1800 bp) but no bands should be observed when PCR is performed with the cwp2-rev primer (3, 4). On the contrary, a clear band of about 800 bp should be observed when running PCR with the wild type strain and the cwp2-rev primer. This corresponds to the result on gel which indicates that the ''CWP2'' deletion was successful.]] | ||
| - | + | ==Lyticase assay== | |
A lyticase assay was performed primarily in order to check the activity of lyticase. Another goal was to compare the rapidity of the cell wall degradation between the IMFD-73 and the [IMFD-73 Δ''cwp2''::kanMX] strain. Lyticase was added to the cells and the cell wall degradation (i.e. protoplast formation) could be displayed by adding SDS and then measuring OD at 600 nm (SDS leads to lysis of protoplasts, while cells with intact cell wall remain unaffected). SDS was added and OD values were taken at different time points. The two graphs (Figure 6 and 7) below show the decrease of the amount of intact cells in % over a time period of 1h with two different amounts of lyticase. The percentages of intact cells were calculated as OD<SUB>600</SUB>(t=x)/OD<SUB>600</SUB>(t=0)*100. One can observe that a slightly smaller amount of cells of the deletion strain were intact between 30 and 60 minutes (except for one outlier at 50 min) which means that the cell wall degraded quicker in the [IMFD-73 Δ''cwp2''::kanMX] strain. This lead to the conclusion that the cell wall is somewhat weakened in the strains with the deleted cell wall mannoprotein gene ''CWP2''. | A lyticase assay was performed primarily in order to check the activity of lyticase. Another goal was to compare the rapidity of the cell wall degradation between the IMFD-73 and the [IMFD-73 Δ''cwp2''::kanMX] strain. Lyticase was added to the cells and the cell wall degradation (i.e. protoplast formation) could be displayed by adding SDS and then measuring OD at 600 nm (SDS leads to lysis of protoplasts, while cells with intact cell wall remain unaffected). SDS was added and OD values were taken at different time points. The two graphs (Figure 6 and 7) below show the decrease of the amount of intact cells in % over a time period of 1h with two different amounts of lyticase. The percentages of intact cells were calculated as OD<SUB>600</SUB>(t=x)/OD<SUB>600</SUB>(t=0)*100. One can observe that a slightly smaller amount of cells of the deletion strain were intact between 30 and 60 minutes (except for one outlier at 50 min) which means that the cell wall degraded quicker in the [IMFD-73 Δ''cwp2''::kanMX] strain. This lead to the conclusion that the cell wall is somewhat weakened in the strains with the deleted cell wall mannoprotein gene ''CWP2''. | ||
[[File:Lyticaseassay1.jpg|thumb|500px|center|Figure 4: Assessment of cell wall degradation by lyticase (50 µl enzyme solution). Prior to the each OD measurement, SDS was added. The percentage of intact cells was calculated as OD<SUB>600</SUB>(t=x)/OD<SUB>600</SUB>(t=0)*100.]] | [[File:Lyticaseassay1.jpg|thumb|500px|center|Figure 4: Assessment of cell wall degradation by lyticase (50 µl enzyme solution). Prior to the each OD measurement, SDS was added. The percentage of intact cells was calculated as OD<SUB>600</SUB>(t=x)/OD<SUB>600</SUB>(t=0)*100.]] | ||
[[File:Lyticaseassay2.jpg|thumb|center|500px|Figure 5: Assessment of cell wall degradation by lyticase (20 µl enzyme solution). Prior to the each OD measurement, SDS was added. The percentage of intact cells was calculated as OD<SUB>600</SUB>(t=x)/OD<SUB>600</SUB>(t=0)*100.]] | [[File:Lyticaseassay2.jpg|thumb|center|500px|Figure 5: Assessment of cell wall degradation by lyticase (20 µl enzyme solution). Prior to the each OD measurement, SDS was added. The percentage of intact cells was calculated as OD<SUB>600</SUB>(t=x)/OD<SUB>600</SUB>(t=0)*100.]] | ||
| - | |||
='''Introduction of indigo synthesizing genes'''= | ='''Introduction of indigo synthesizing genes'''= | ||
Revision as of 09:43, 25 September 2012
Contents |
Results Summary
Spot test analysis and growth measurements of yeast in urine were performed in order to test the survival of yeast cells in urinary medium. These initial experiments showed that yeast cells are able to survive in urinary medium which is an important prerequisite for the biosensor.
The gene CWP2, encoding a mannoprotein in the cell wall was successfully deleted in the yeast strain IMFD-73. The deletion was confirmed by verification PCR. In addition, a lyticase assay showed that [IMFD-73 Δcwp2::kanMX] was degraded faster than IMFD-73 and this leads us to the conclusion that the cell wall is somewhat weakened in the strains with the deleted cell wall mannoprotein CWP2.
Two different plasmids both containing the receptor gene LHCGR but one with the human signal peptide and the other one with a yeast signal peptide was created. Both plasmids were successfully cloned into IMFD-73 and [IMFD-73 Δcwp2::kanMX] but in neither strains could the receptor be proved as functional in detection of hCG.
The Indigo group managed to obtain different colour changes in different growth media by introducing a plasmid containing the genes tnaA and fmo. For instance, blue bubbles could be seen in one medium and other media turned into different shades of brown. However, we did not manage to confirm the presence of bio-indigo and to exactly determine which compound or reaction that was responsible for the colourful bubbles or colour changes.
[IMFD-73 Δcwp2::kanMX] was successfully transformed with both receptor genes and genes required for bio-indigo production. However, the system was never functional as a pregnancy test kit since it did not give any significant response in the presence of hCG. More detailed analysis and the discussion of the results and discussion can be found in the sections below.
Survival of yeast in urine
In order to test if the cells remained alive in the urin, 300 µl of each culture (after 4h) were taken and OD600 was adjusted to 0.5. The samples were diluted 3x, 9x, 27x and 81x and 10 µl aliquots of each dilution were spotted on an YPD plate (Figure 2). The cells from both the YPD and the urine media could grow, this means that the cells survived 4 h in urine and grew normal after being spotted on a YPD plate. Summarizing, yeast cells are unable to proliferate in the urine medium but do survive under these conditions.
Deletion of CWP2 gene
One task of our iGEM project was the deletion of the CWP2 gene, which is encoding a cell wall mannoprotein. By removing it, we aimed for higher cell wall permeability and thus enhanced chances of our ligand hCG to pass the cell wall and to bind to the membrane-bound receptor.
Gene Deletion according to the Bipartite method
The gene deletion was performed according to the bipartite method. The results from the first PCR reactions, in which we amplified the overlapping fragments, can be seen in Figure 3.

The next step was to fuse together the overlapping fragments. The results of the fusion PCR are shown in the Figure 4. By transforming yeast with these fragments, homologous recombination should occur which will lead to the exchange of the CWP2 gene with the kanMX cassette.

Colony PCR
Yeast strain IMFD-73 was transformed with 150 ng of each fragment and grown on G418 plates. Colonies could be observed, which were purified, and colony PCR was then performed in order to test whether the kanMX cassette was integrated randomly in the genome or had replaced the CWP2 gene as desired. PCR was run with a forward primer outside of the inserted region and reverse primers within the CWP2 gene or kanMX cassette respectively. As a control, a wild-type strain and a Δcwp2 strain from a deletion library were also tested. The results of the PCR reactions indicated that the deletion was correct (see Figure 5).

Lyticase assay
A lyticase assay was performed primarily in order to check the activity of lyticase. Another goal was to compare the rapidity of the cell wall degradation between the IMFD-73 and the [IMFD-73 Δcwp2::kanMX] strain. Lyticase was added to the cells and the cell wall degradation (i.e. protoplast formation) could be displayed by adding SDS and then measuring OD at 600 nm (SDS leads to lysis of protoplasts, while cells with intact cell wall remain unaffected). SDS was added and OD values were taken at different time points. The two graphs (Figure 6 and 7) below show the decrease of the amount of intact cells in % over a time period of 1h with two different amounts of lyticase. The percentages of intact cells were calculated as OD600(t=x)/OD600(t=0)*100. One can observe that a slightly smaller amount of cells of the deletion strain were intact between 30 and 60 minutes (except for one outlier at 50 min) which means that the cell wall degraded quicker in the [IMFD-73 Δcwp2::kanMX] strain. This lead to the conclusion that the cell wall is somewhat weakened in the strains with the deleted cell wall mannoprotein gene CWP2.
Introduction of indigo synthesizing genes
Expression of the gene for the LH/CG receptor in yeast
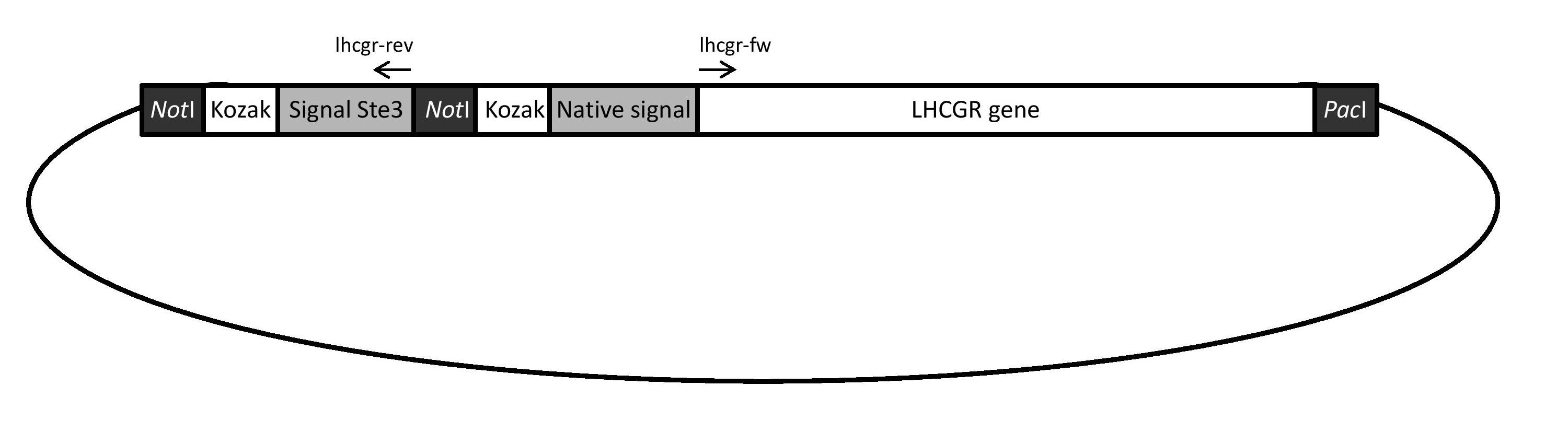
Two different versions of the LH/CG receptor were to be evaluated and expressed in yeast during this project: one with the original human signal peptide fused to the receptor and one with the yeast Ste3 signal peptide fused to the receptor. Both versions were obtained using the gene construct illustrated in Figure 24. The construct was ordered from and synthesized by GenScript.
Obtaining and cloning the yLHCGR and the hLHCGR gene
The yeast signal peptide version of the receptor gene, hereafter referred to as “yLHCGR”, was obtained with PCR using the original gene construct (Figure 24) and 5’ phosphorylated primers (lhcgr-rev and lhcgr-fw), as can be seen in Figure 25 A. The yLHCGR construct was then religated and when cut with restriction enzymes NotI and PacI and run on a gel, the obtained band displayed a correct length of approximately 2100 bp (see Figure 25 C). The band was cut out and the yLHCGR gene was then ligated into the shuttle plasmid pSP-GM1 and amplified in E. coli.
The human signal peptide version of the receptor gene, hereafter referred to as “hLHCGR”, was obtained by digesting the original gene construct with NotI and PacI (Figure 25 B). Gel electrophoresis was run and the obtained fragment displayed a correct length of approximately 2100 bp (see Figure 25 C). The band was cut out, ligated into the pSP-GM1 plasmid and amplified in E.coli.
Saccharomyces cerevisiae strains IMFD-70, IMFD-72, IMFD-73 and IMFD-74 were then successfully transformed with the yLHCGR and hLHCGR expression plasmids respectively. All these strains have been optimized for the coupling of human GPCRs to the pheromone signaling pathway and they differ in their expression of different G alpha proteins. IMFD-70 has the endogenous G alpha protein [KÄLLA] while the other strains express different versions of yeast/human chimeric G alpha proteins. These chimeric proteins have had the five last amino acid residues of the C terminal end replaced with the corresponding human amino acids [Unpublished data]. IMFD-73 has a chimeric alpha subunit of such type that it should have the best chances of interacting with the LH/CG receptor and was therefore the strain that was mainly used during this project. All strains were kindly provided by the Kondo group at Kobe University, Japan.
Sequencing
In order to confirm that the yLHCGR gene and the hLHCGR gene not only have the correct length but also the correct sequence, the yLHCGR and the hLHCGR plasmid were sent in for sequencing. Results indicated that the sequences were correct for both genes.
Evaluating the functionality of the receptor
The ability of the receptor to detect hCG (i.e to bind the hormone and transmit the signal) when expressed in yeast, was evaluated several times under different conditions. Initial tests were performed by screening for fluorescence after the addition of hCG to the yeast cells. A final test also included screening for indigo production in the completed biosensor system.
Initial tests: Screening for GFP expression
The yeast strains that have been transformed with the gene for the LH/CG receptor have the gene for GFP placed under the control of the pheromone-induced FIG1 promoter. An initial test to see if the LH/CG receptor could detect hCG and activate the pheromone pathway, was therefore done by screening for fluorescence. Below, results from three of these screens are presented.
IMFD-70, IMFD-72, IMFD-73 and IMFD-74, grown at 30°C
One experiment was carried out using all four, IMFD-70, -72, -73 and -74 strains, transformed with yLHCGR, hLHCGR and the empty pSP-GM1 plasmid respectively. Cells were cultivated at 30°C and were treated with lyticase in order to degrade the cell wall. 200 IU of hCG was then added to each strain. Screening for fluorescence was done using a fluorescence microscope. See Figure 3, 4, 5 and 6 for pictures that have been selected as representative of all results obtained during this experiment.
Results obtained for the cells not treated with hCG and cells transformed with the negative control plasmid, indicate that there is relatively high level of background noise of GFP expressions in all IMFD strains. Generally, the fluorescence intensity is approximately equal between samples that have had the hCG hormone added to them and samples that did not have hCG added to them. It was therefore concluded that the LH/CG receptor, when expressed in these yeast strains and tested under the given conditions, is unable to detect hCG or at least that the receptor it is not functioning in a way that produces a sufficiently strong GFP output signal to be distinguished from the background noise.
IMFD-73, grown at room temperature
In a second test, the transformed IMFD-73 strain with either the yLHCGR, hLHCGR or the empty pSP-GM1 plasmid, were cultivated at room temperature. Gron wth at lower temperatures is often used in heterologous protein production since it may improve protein folding. Cells were then treated with cell wall-degrading enzyme lyticase and 200 IU of hCG was added. Results from the fluorescence microscope can be seen in Figure 30, 31 and 32.
Once again, high levels of background fluorescence were present. Results indicated that also when cells are grown at room temperature, the LH/CG receptor is unable to detect hCG or uncapable to produce an output signal that can be distinguished from the background noise in response to detecting hCG.
IMFD-73-ΔCWP2, grown at 30°C
In a third test, the deletion strain IMFD-73-ΔCWP2 transformed with yLHCGR, hLHCGR and the empty pSP-GM1 plasmid respectively was used. The IMFD-73-ΔCWP strain potentially has a weakened cell wall. Cells were grown at 30°C and were not treated with lyticase before the addition of 200 IU hCG. Fluorescence microscope pictures can be seen in Figure 33, 34 and 35.
As previously, the yeast transformed with the LH/CG receptor gene and that had the hCG hormone added to them, did not produce an output signal that could be distinguished from the fluorescence intensity of cells that did not have hCG added to them or that did not contain the LH/CG gene.
Final test of the completed system: screening for color change
One last test was performed using the completed biosensor system: IMFD-73-ΔCWP2+pFig-Indigo+hLHCGR or yLHCGR. The cultures were grown over night before adding hCG in the morning. 3 hours later more hCG was added. From the colorometic assay (see "Results: indigo"), it was concluded that it takes approximately 7 hours for the pIndigo cells to produce a visible color change when grown at 30°C. However, 7 hours after adding hCG to the finalized system, no color change could be observed in any of the cultures (see Figure 36 and 37) and it was concluded that the biosensor did not succeed in detecting the hormone.
 "
"