Team:Peking/Project/Communication/Design
From 2012.igem.org
Zhangzidong (Talk | contribs) |
Zhangzidong (Talk | contribs) |
||
| Line 52: | Line 52: | ||
</p> | </p> | ||
<div class="floatC"> | <div class="floatC"> | ||
| - | <img src="" alt="Fig 2. Device" /> | + | <img src="/wiki/images/2/23/Peking2012_communication_setup.png" alt="Fig 2. Device" style="width:500px;"/> |
<p class="description">Fig 2. Set-up for Light Communication</p> | <p class="description">Fig 2. Set-up for Light Communication</p> | ||
</div> | </div> | ||
Revision as of 13:17, 21 September 2012
Now that we can enable bacterial cells to respond to dim light, which implies that cell-cell communication through light has been made a possibility. We are looking forward to implementing it.
Light Emitting Cell
The first concern was what kind of light emitting cell we should choose. It has to meet several requirements:
- 1) The light emitted should have a maximum intensity of approximately 480nm, which is the maximum absorption wavelength of VVD.
- 2) The cell must require no excitation to emit light.
- 3) The cell must be able to emit light at 30oC, which is the functional temperature of our light sensitive repressor LexA-VVD.
- 4) The light needs to be strong enough to be detected by our Luminesensor.
After browsing through scientific literature, we chose the bacteria luciferase (lux system), which originated from Vibrio fischeri. It is expected to emit blue-green light with a maximum intensity at 490nm. The light emitting reaction only requires bacteria metabolic substrates like FMNH2, O2 and fatty aldehyde, which means we would not have to add exogenous substrates into the system. Also, it was reported that the best temperature for E. coli transformed with lux genes to emit light is 30oC.
Fortunately, we found an already existing biobrick in the Parts Registry constructed by Cambridge 2010 iGEM team – luxbrick (BBa_K325909). They have successfully optimized the codon for expression and transformed the lux system into E. coli. In this biobirck, the light emitting related genes are placed under the pBAD promoter, and the cells are able to produce relatively strong blue-green light under the induction of L-arabinose. Therefore, we decided to use this part to construct light emitting cell for bio-luminescence communication.

Fig 1. TOP10 cells transformed with luxbrick which is a biobrick constructed by Cambridge 2010 iGEM team
Device
The next issue we placed our focus on is how to build a device to enable cells to talk through light in an easily observable way. To obtain this goal, we first had to solve some problems:
- 1) As the intensity of bacteria luminescence may not be high enough, we had to place light emitting cells and the sensing cells in close proximity. However, it has better not mix the cells together due to the need to observe the reaction of the sensor cells separately.
- 2) The light emitting reaction highly depended on the availability of O2 in the environment. Therefore, our means of maintaining the concentration of O2 was an important issue.
- 3) As our light sensing cell is very sensitive, which has been shown in the characterization of our Luminesensor, it’s important to exclude the influence of other sources light.
To solve the problem of O2 maintenance, we decided to put the light emitting cell in a conical flask and keep fresh air pumping into the culture. We put a test tube containing light sensing cell in the conical flask, immersing the sensor cell culture into the light emitting cell to keep them in close proximity. To exclude the influence of the other sources of light in the environment, the whole set-up was wrapped tightly with aluminum foil. Finally, the whole set-up was kept on a shaking incubator at 30oC.
The set-up is shown in the figure below:
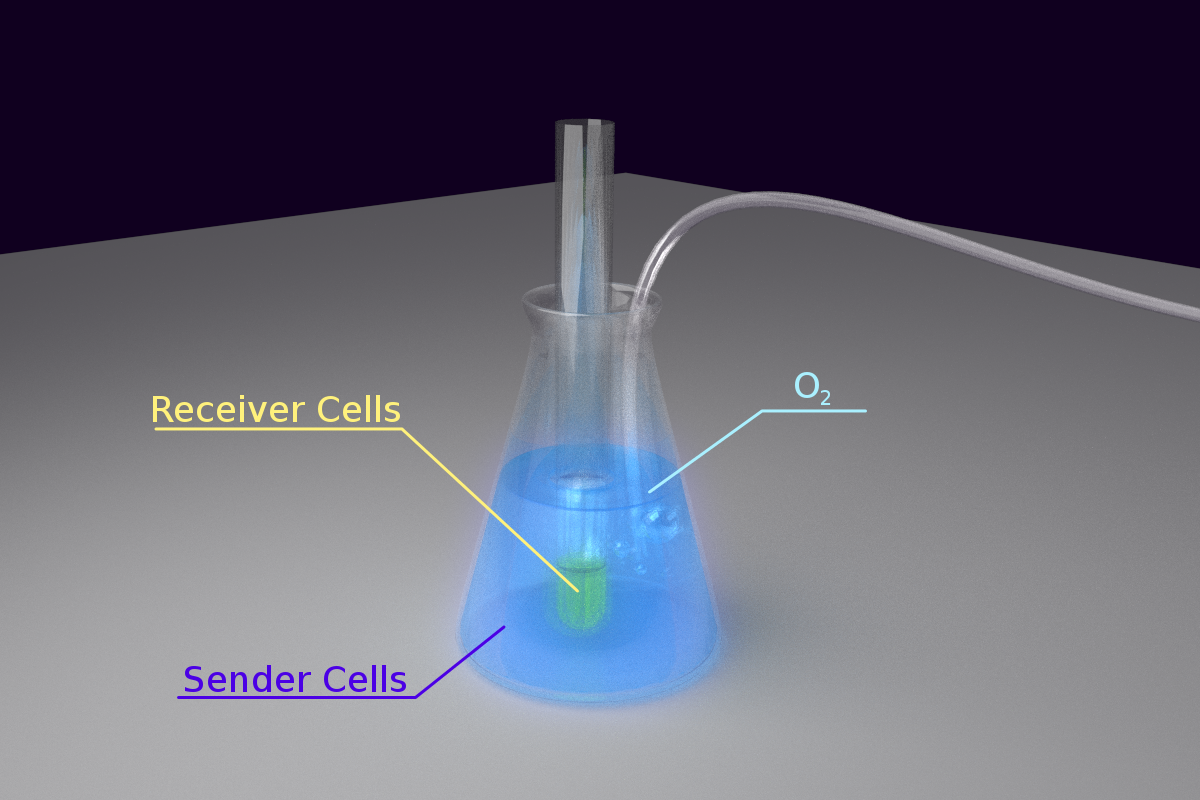
Fig 2. Set-up for Light Communication
Light-On system
Fig 3. Light-on System upgraded from Luminesensor
As Luminesensor is a photo-activated repressor, forming a light-off system. We also want to construct a separate light-on system, which can produce more direct and visualized results than the light-off system, especially during bio-luminescence communication.
However, it is difficult to create another fusion transcription factor similar to LexA-VVD to realize "light-on" in E. coli because there are hardly any transcription activators that works as LexA in E. coli. Thus, we decided on a simpler method. We decided to build a gene circuit based on our light-off part by adding an inverter, realizing the "light-on" System.
As shown in the diagram, tetR under the control of recA promoter is repressed by LexA-VVD, and the reporter (GFP here) under PtetR promoter is repressed by tetR. So blue light will repress tetR, thereby decreasing the repression on GFP, forming a "light-on" system.
 "
"