Team:Frankfurt/New Yeast RFC
From 2012.igem.org
(→Gap repair cloning for iGEM) |
(→Gap repair cloning for iGEM) |
||
| Line 38: | Line 38: | ||
Although the restriction sites of the Biobrick pre- and suffix are unimportant in our context, the biobricking of genes leads to possiblity to amplify all Biobricks with the same prefix and suffix type with the same PCR primers. There are already some PCR primers in the Registry which anneal at the pre- or suffix sequence. By adding an additional non annealing sequence to the primer a desired overlap can be produced on every Biobrick.<br> | Although the restriction sites of the Biobrick pre- and suffix are unimportant in our context, the biobricking of genes leads to possiblity to amplify all Biobricks with the same prefix and suffix type with the same PCR primers. There are already some PCR primers in the Registry which anneal at the pre- or suffix sequence. By adding an additional non annealing sequence to the primer a desired overlap can be produced on every Biobrick.<br> | ||
| - | The further idea of our project would be to create DNA fragments suitable for gap repair cloning. It would be great if the assembly and cloning of genes in yeast become simpler than the cloning by restriction and ligation. In the following we want to outline the basic idea. <br>Any expression plasmid have a similiar ordered insert region. Depending on the number of genes on the plasmid the insert region consists of several repeats of the general scheme promoter-gene-terminator. Gap repair suitable DNA now can simply be created by choosing promoters, terminators and genes from the registry. Thus one can amplify the genes with primers which contain overlaps to the end of the promoter and the beginning of the terminator. With such PCR products it is possible to do the homologue recombination in yeast. Since any Biobrick (from the same standard) can be amplified with the same primer it is sufficient to construct two standard primers for every promoter and terminator in the registry, one | + | The further idea of our project would be to create DNA fragments suitable for gap repair cloning. It would be great if the assembly and cloning of genes in yeast become simpler than the cloning by restriction and ligation. In the following we want to outline the basic idea. <br>Any expression plasmid have a similiar ordered insert region. Depending on the number of genes on the plasmid the insert region consists of several repeats of the general scheme promoter-gene-terminator. Gap repair suitable DNA now can simply be created by choosing promoters, terminators and genes from the registry. Thus one can amplify the genes with primers which contain overlaps to the end of the promoter and the beginning of the terminator. With such PCR products it is possible to do the homologue recombination in yeast. Since any Biobrick (from the same standard) can be amplified with the same primer it is sufficient to construct two standard primers for every promoter and terminator in the registry, one overlapping with the front end and one with the rear end of the promotor. We want to design two standard primers for any of the promoters and terminators we used and for those which can already be found in the registry. |
|} | |} | ||
Revision as of 08:16, 21 September 2012
| Home | Team | Project | Organisms | New Yeast RFC | Notebook | Registered Parts | Modeling | Safety | Attributions | Official Team Profile |
|---|
Contents |
The benefit of vector assembly in yeast
Introduction
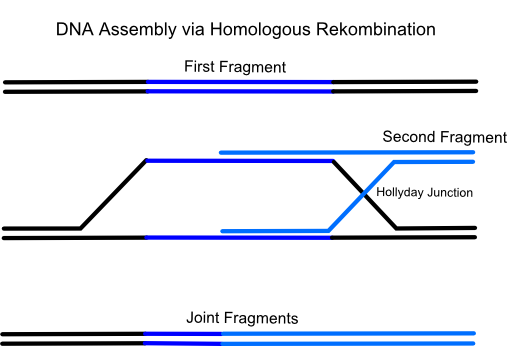
Gap repair cloning is an elegant method for vector construction. It takes advantage from the homologous recombination system of Sacchaomyces cerevisiae (common yeast) which has a heightened activity. What does this mean in detail? There are many endogenous and exogenous factors (for example reactive oxygen-species, ionizing radiation, chemicals and failing of DNA binding enzymes (e.g. collapsed replication forks)) which causes DNA double strand breaks. For the cell this is the most dangerous DNA damage because even if it occurs in rather unimportant regions the cell will not survive the next cell cycle. That's the reason why yeast possesses enzymes which have the ability to repair a broken double strand by pairing it with a very similiar DNA region (typically on the homologous chromosome). This process is called homologous recombination. Using the gap repair method this natural process can be exploited for the construction of large cloning vectors in yeast.
Design of DNA fragments for gap repair cloning
The idea of the method is to transform a series of linear, successive DNA fragments into one yeast cell. The linear fragments have open blunt ends like they occur after a double strand break. If a homologous sequence is available it will be treated like a genomic double strand break and homologous recombination takes place. When the successive DNA fragments are designed in a specific way which includes large sequence overlaps to the respectively following fragment yeast will recombinate them together.For the formation of a cloning vector the first fragment is a yeast-E.coli shuttle plasmid which is linearized by an appropriate restriction digest. A shuttle plasmid is a plasmid which is stable both in yeast and in Escherichia coli. The first fragment of the insert has to possess an homologous overlap to both the wished insertion site on the plasmid and to the beginning of the second fragment. The end of the second fragment has to possess an overlap to the beginning of the third one and so on. At least the end of the last fragment of the insert again has to possess an overlap homologous to the second insertion site on the plasmid.
At the lab of our instructor up to eighteen single fragments were assembled in a single transformation. Another advantage of the method is that no scars are left between the inserted fragments. Assembly of fragments to joint genes is possible. Restriction enzymes only have to be used once for linearization of the shuttle plasmid.
 "
"