Team:Calgary/Notebook/Hydrocarbon
From 2012.igem.org
| Line 70: | Line 70: | ||
<h3>Desulfurizationtion</h3> | <h3>Desulfurizationtion</h3> | ||
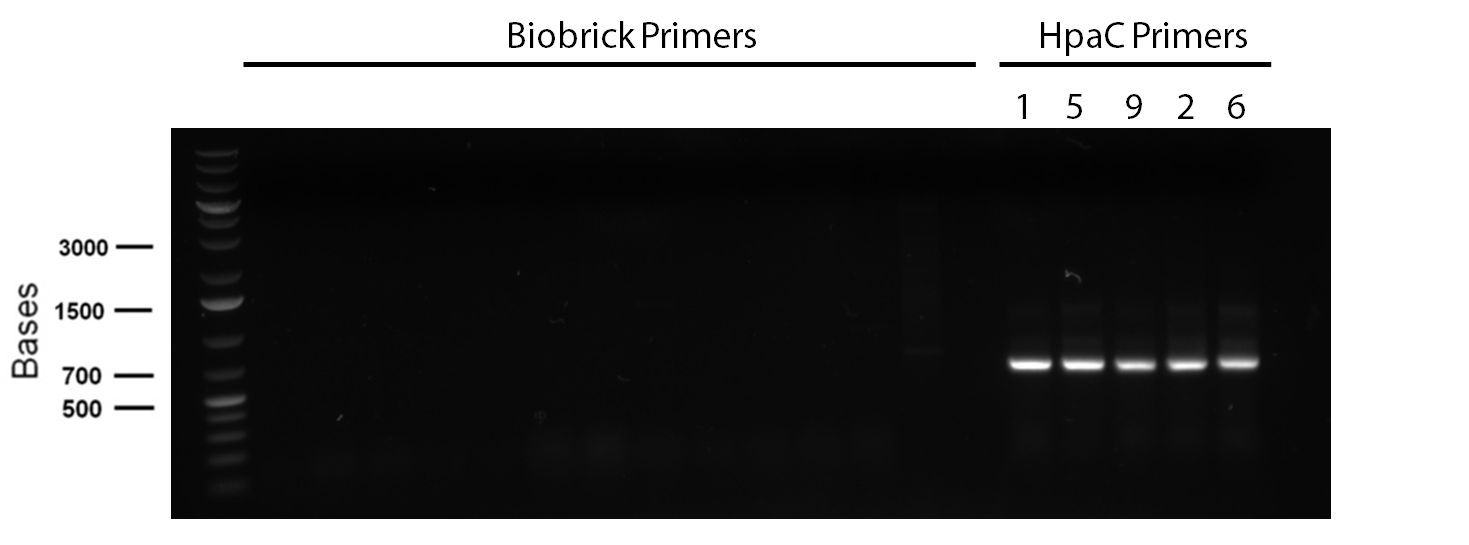
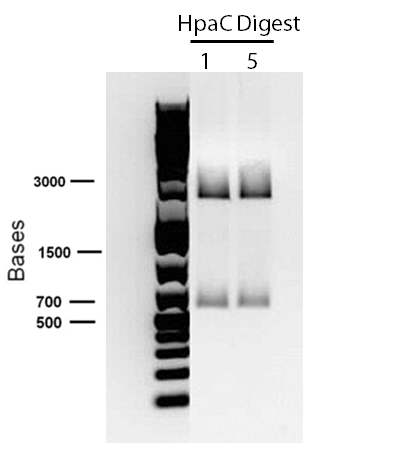
| - | </p> in order to confirm the hpaC biobrick construction, first we did two sets of colony PCR(choosing white colonies from the 3 plates we grew last week). One with hpaC primers and one with biobrick primers. After running them on the gel we saw equal bands for the PCRs performed using hpaC bands(However, a PCR using biobrick primers was performed later and the same results were obtained). Colonies 1(-) and 5(-) were used to make overnight cultures with. Then the overnight cultures were used | + | </p> in order to confirm the hpaC biobrick construction, first we did two sets of colony PCR(choosing white colonies from the 3 plates we grew last week). One with hpaC primers and one with biobrick primers. After running them on the gel(first picture below) we saw equal bands for the PCRs performed using hpaC bands(However, a PCR using biobrick primers was performed later and the same results were obtained). Colonies 1(-) and 5(-) were used to make overnight cultures with. Then the overnight cultures were used for miniprep. Digestions were performed on the miniprep products using EcoRI and Pst. The results were good(second picture below) two bands were observed on each colomn(one for vector and the other for hpaC). </p> |
</html>[[File:UCalgary2012_04.06.2012-desulfurisation_hpacverification.jpg|thumb|900px|center]] | </html>[[File:UCalgary2012_04.06.2012-desulfurisation_hpacverification.jpg|thumb|900px|center]] | ||
[[File:Ucalgary2012 06.06.2012-digestion of hpaC with E and P.jpg|thumb|850px|center]]<html> | [[File:Ucalgary2012 06.06.2012-digestion of hpaC with E and P.jpg|thumb|850px|center]]<html> | ||
</html> | </html> | ||
Revision as of 19:02, 15 June 2012
Week 1 (May 1-4)
Week 2 (May 7-11)
Decarboxylation
For the decarboxylation sub-project, the second week was entirely focused on literature research and the practice of basic laboratory techniques. 8 potential pathways were identified as potential candidates for naphthenic acid decarboxylation. The first of these would utilize only the University of Washington's "PetroBrick" (from iGEM 2011), consisting of the genes encoding the enzymes acyl-ACP reductase (AAR) and aldehyde decarbonylase (ADC). We have planned to verify the PetroBrick in the distribution plates and test its efficacy on naphthenic acids in the coming weeks. If this proves to be unsuccessful, we will begin investigating the alternative approaches, beginning with replacing AAR with carboxylic acid reductase (CAR) from Nocardia iowensis, a very unspecific reductase shown to work on structures resembling naphthenic acids. Failing this, the remaining pathways will be examined; however, the disadvantage in these pathways is their direct reliance on the success of the other steps, as the naphthenic acids must be degraded to the point of resembling branched-chain fatty acids (since all remaining pathways are related to fatty acid metabolism).
Denitrification
In the first two weeks of iGEM our group has focused on reviewing literature regarding the bioremediation of nitrogen groups attached to naphthenic acids. The most prevalent N heterocycle is carbazole, representing 75% of total nitrogen by mass. The upper pathway of carbazole biodegradation is catalyzed by the enzymes coded for by the car operon, CarA (CarAaAbAd), CarB (CarBaBb), and CarC. These enzymes convert carbazole to anthralinic acid. The lower pathway is catalyzed by the enzymes of the ant operon, antA, B, and C, yielding cathecol while releasing CO2 and NH3. The car and ant operons are both regulated by the Pant regulator which is induced by the protein, antR. CarAa also has its own promoter which is not induced by antR. We have also investigated an alternative pathway using CarA combined with an amidase (amdA) that selectively cleaves NH2 from an intermediate of the car pathway. This could bypass much of the car/ant pathway and is possibly more efficient.
We have decided to use Pseudomonas resinovorans and Rhodococcus erythropolis to amplify these genes from. CarABC and AntABC from P. resinovorans has been shown to have a wide range of nitrogen containing substrate specificity. R. erythropolis contains the amdA gene that we wish to use, and some evidence suggests that it may also be able to degrade sulfur rings through its CarABC pathway.
In addition to our research we have also been learning some of the lab techniques we will be using this summer. This includes transforming a plasmid into E. coli, plating and selecting for bacteria containing the plasmid, verifying with colony PCR, performing a mini-prep and a restriction digest.
Desulphorization
Things we did.
Ring Cleavage
This week we mainly researched aromatic ring cleaving using intra- and extradiol dioxygenases from species of Pseudomonas and Bacillus but also began literature searches on aliphatic ring cleavage done by monooxygenases.
Week 3 (May 14-18)
Decarboxylation
The third week included some additional literature investigation in the first two days. The iGEM distributions arrived this week, and verification began on May 17th by transformation into E. coli, followed by colony PCR on May 18th (using standard protocols on the wiki). Additionally, primers were designed for CAR in N. iowensis, along with primers for Nocardia posphopantetheine transferase (NPT), a second enzyme required for optimal function in the former, and a short list of contacts were acquired to request the donation of the required strain (called NRRL 5646) from researchers who have worked with it previously. A sort of form email was drafted for this purpose, and should this be unsuccessful, we will be purchasing the strain from DSMZ (http://www.dsmz.de). In the following week, we will begin with development of overnight cultures and gel preparation.
Ring Cleavage
In the second week we continued to do more research on the degradation of alicyclic compounds and found two strains of bacteria that contained genes needed for this process. The first strain, Thauera butanivorans, contains the genes required to activate the ring by adding a hydroxyl group. The gene is called butane monooxygenase and is composed of three subunits, a hydroxylase, a reductase, and a regulatory component. The second strain, Acinetobacter sp. SE19, contains the genes needed to oxidize the alcohol, formed by butane monooxygenase, and to cleave the ring. There is a cluster of nine genes that perform this oxidation and cleavage but only six are involved directly.
Week 4 (May 22-25)
Decarboxylation
This week, overnight cultures were grown from the colonies prepared in Week 3. Sigma compounds - for the purpose of testing the PetroBrick on naphthenic acid analogues - were selected from the list provided. The compounds to be used include cyclohexanepentanoic acid, cycohexane-1,1-dicarboxylic acid, and benzo[b]thiophene-3-acetic acid. A mini-prep was completed on the overnight cultures prepared earlier in the week, according to protocol obtained from the wiki. The DNA concentration in the resulting samples was measured by nanodrop to confirm successful plasmid extraction. A restriction digest was completed also, but it was not run on a gel this week; this was left for Week 5.
Desulfurization
This week we devised a protocol for biobricking the hpaC and started our lab work. The PCR performed on dry hpaC containing plasmids was not successful so we have to repeat the PCR. We also need to transform E.coli with these plasmids in the next week in order to make sure we wont lose all the plasmids in case the PCR was not successful again. We ordered the substrates we are going to use. Once the substrates and the Rhodococcus strain arrive we are going to test how effectively the bacteria can desulfurize different compounds.
Denitrification
This week we reviewed the primers listed in the database and also designed some new ones. Primers for CarAa, CarAc, and CarAd were designed individually, while primers for CarBaBbC and AntABC were designed to encompass multiple genes in a sequence. These primers were designed to be used on Pseudomonas putida which we decided to use as our gene source since it was available to us as opposed to ordering Pseudomonas resinovorans. We also designed a primer for the AmdA gene from Rhodococcus erthyroplois. In addition to ordering these primers we also ordered the nitrogen containing compounds that we will need to test these enzymes on. We decided on using carbazole to make sure the enzymes can perform their natural function as well as pyrrolidine to test them on a similar ring structure. We also ordered cyclohexamine in order to independently test the function of the alternative AmdA pathway. Finally, we decided to eventually order 4-Piperidine butyric acid hydrochloride to test how the enzymes will work on nitrogen containing naphthenic acids. However, we decided since it is a very expensive compound we would wait to make sure the enzyme's work on more simple compounds before ordering it.
We also started our work in the wet lab by plating colonies and making an overnight culture of -80 freezer stock Psuedomonas putida on Thursday. We also resuspended the primers for CarAa, CarAc, CarAd, AntA, AntB, and Ant C that were already in the database. We were able to use the colonies that grew on the streak plates to start a colony PCR to attempt to isolate each of these genes on Friday.
Ring Cleavage
We started looking into more organisms that can carry out cyclohexane degradation and found one called Brachymonas petroleovorans. This organism is capable of both hydroxylating cyclohexane and carrying out cyclohexanol oxidation. We are also hoping to find evidence that Pseudomonas fluorescens, Pf-5 has genes that can cleave aliphatic rings, however, first we have to determine if Pf-5 can grow in the presence of butylcyclohexane. To do so we carried out an assay, using LB, testing the toxicity of butylcyclohexane on Pf-5. After 24 hours of incubation we took OD600 readings¬ which suggested that the presence of butylcyclohexane did not have effect on cell growth. We also looked up Pseudomonas fluorescens Pf-5 in the Pseudomonas Genome Database and found genes that code for proteins with similar functions to what we have found for aromatic and aliphatic ring cleavage.
Bioinformatics/Modelling
Handy bioinformatics tools
FMM (From Metabolite to Metabolite) is a critical tool for synthetic biology. FMM can reconstruct metabolic pathways form one metabolite to the other one. http://fmm.mbc.nctu.edu.tw/
This webpage runs the software that performs the enumeration of all pathways for target compounds in KEGG. More precisely, the desired KEGG compound is submitted in order to get the map of all pathways connecting the compound to metabolites endogenous to E. coli. Optionally, the full map containing the supplements can also be requested. http://bioretrosynth.issb.genopole.fr/tools/metahype/
DNA Designer 2.0 is a Drag and drop standalone software, runs on Windos, MacOS. https://www.dna20.com/secure/order.php?page=genedesigner2
DNADesign is a Web-based application http://genedesign.thruhere.net/gd/
Modelling
Constraint-based flux analysis - modeling microorganism metabolic network and perform flux analysis.The metabolic network is translated in to a matrix and then the simulation will calculate a pathway that optimizes the goal (max biomass or max production). The network will be reconstructed (gene/reaction can be added or deleted) to enhance the expected pathway to degrade target compounds. The media (compounds, thermo-conditions) will be manipulated to find the best condition(s) that satisfy the microorganisms to digest the target compounds and reach a good growth rate (say above threshold).
Week 5 (May 28 - June 1)
Decarboxylation
None of the subproject members were present on Monday of this week (May 28). On Tuesday (May 29), a gel was run on the restriction digests of the extracted plasmids from Week 4, which appeared to confirm the successful transformation of the PetroBrick, showing clear bands at the pertinent location (approx. 2300 base pairs). Based on the apparent success of the gel, the products were sent away for sequencing. Stocks were prepared for the alkane production medium outlined in University of Washington's PetroBrick protocols from 2011 (see https://2011.igem.org/Team:Washington/alkanebiosynthesis). The medium itself is to be prepared in Week 6 once the results of sequencing are (hopefully) acquired.
Ring Cleavage
This week we redid the previous assay with Pf-5 and butylcyclohexane using glass vials instead of falcon tubes. These vials allowed for more surface and better containment of the volatile compound. We carried out this assay using both LB and M9-MM. After 24 hours we took OD600 readings and obtained similar results as last week. The growth was considerably decreased in the M9-MM, however we are not sure whether the bacteria were able to use the compound as a carbon source because the MM contained glucose. The fact that the growth decreased showed that they might depend on the glucose in the media to grow.
Denitrification
On Monday and Tuesday we used the database primers to attempt to isolate CarAa, CarAc, CarAd, AntA, AntB, and AntC from the Psuedomonas putida we plated last week. CarAd, AntB, and AntC were all put in the gradient PCR machine to account for the wide range of their primers’ melting points, while the others were done via regular PCR. Unfortunately, no positive results were obtained from these reactions. The PCR on CarAc and AntA was repeated on Wednesday, still not giving positive results. Finally, we attempted to use a salt concentration gradient on the PCR reaction for CarAc and AntA, using concentrations that ranged from 1.0 microlitres/tube to 2.0 microlitres/tube in increments of 0.2 microlitres/tube. This helped as CarAc showed bands in samples that had concentrations of 1.2 and 1.4 microlitres/tube. AntA also showed bands, however they were not the correct size, indicating contamination and/or non-specific annealing of the primer. The positive control also showed bands of the correct size. Earlier in the week we also made overnight cultures of 3 environmental strains (28, 29, 30) of Psuedomonas putida from -80 glycerol stock. On Friday we performed a genomic prep on these cultures, and plan on attempting to amplify genes from the isolated DNA next week.
Desulfurization
Since we wanted to make sure we would not run out of pUC18(plasmid containing hpaC gene), we transformed some E.coli cells with it. We grew them on plates containing A, K, T and C antibiotics and they only grew on A. Therefore pUC18 has A resistance. We did a three sets of PCR with hpaC primers, one using 1/10 dilution of pUC18, the other using 1/100 dilution of pUC18 and one with the colonies we had just obtained by transforming the E.coli cells with pUC18. The PCR worked and we saw bands of the same size for all three sets of PCR. (Unfortunately, the picture we saved is not a good one since some of the bands faded awayunder UV). Then we did a PCR purification to obtain the pure hpaC gene. We also did 3 sets of digestion(using pairs of X&P enzymes, E&S enzymes and E&P enzymes) to insert the hpaC gene into the pSB1c3 vector. All the sets grew successfully.
Week 6 (June 4 - June 8)
Denitrification
We started off this week by determining the DNA concentration of our genomic prep samples from last week using the nanodrop. DNA concentration for all three putida strains was at least 1000 ng/microlitre, well above what was needed for PCR. 1/2 and 1/3 dilutions were prepared for all three strains so as not to have an excess of template DNA in PCR reactions. PCR was performed on all 3 strains using primers for CarAc, CarAd, AntA, AntB, and AntC using 6 replicates per gene. The only successful amplification appeared to be AntB and CarAc, both from strain 28 (with weaker bands in strain 29). We then performed another PCR, just on those two genes with an increased amount of Taq polymerase to hopefully get enough amplified DNA to move forward with. This resulted in strong bands for both at the expected size. We then performed PCR purification using the Qiaquick kit and obtained samples containing 33.5 ng/microlitre of AntB DNA and 129 ng/microlitre of CarAc DNA. These concentrations were both sufficient to begin a restriction digest and ligation of these parts into vector PSB1C3. Next week we hope to verify the results of the restriction digest, continue to amplify CarAc and AntB from strain 28, and hopefully submit a biobrick for sequencing.
 "
"