Team:LMU-Munich/Data
From 2012.igem.org
Franzi.Duerr (Talk | contribs) |
Franzi.Duerr (Talk | contribs) |
||
| Line 144: | Line 144: | ||
|} | |} | ||
| - | <p align="justify">Tab. 1 shows that after treatment with French Press and ultrasound the number of spores compared to the untreated samples was increased. We assume the reason for this was the impurity of these samples that derived from damaged vegetative cells. Thus, during counting | + | <p align="justify">Tab. 1 shows that after treatment with French Press and ultrasound the number of spores compared to the untreated samples was increased. We assume the reason for this was the impurity of these samples that derived from damaged vegetative cells. Thus, during counting it was not always possible to distinguish between mature spores and cell waste. However, a huge difference in number of vegetative cells and spores between untreated and lysozyme treated samples was noticeable under microscopy as it is visualized in the table above and the pictures below. Because the vegetative cells were lyzed and not damaged it was easy to recognize the mature spores. |
| - | + | ||
Revision as of 15:31, 19 September 2012
The LMU-Munich team is exuberantly happy about the great success at the World Championship Jamboree in Boston. Our project Beadzillus finished 4th and won the prize for the "Best Wiki" (with Slovenia) and "Best New Application Project".
[ more news ]

Data
Here you will find all of our project data. For our big breakthroughs, or to follow specific projects, see the individual project pages:
| +--+--+--+--+--+--+-- | +--+--+--+--+-- | +--+--+--+--+--+-- | +--+--+--+--+--+-- |

Bacillus BioBrickBOX |

SporeCoat FusionProteins |

Germination STOP |
Bacillus BioBrick Box - Promoters
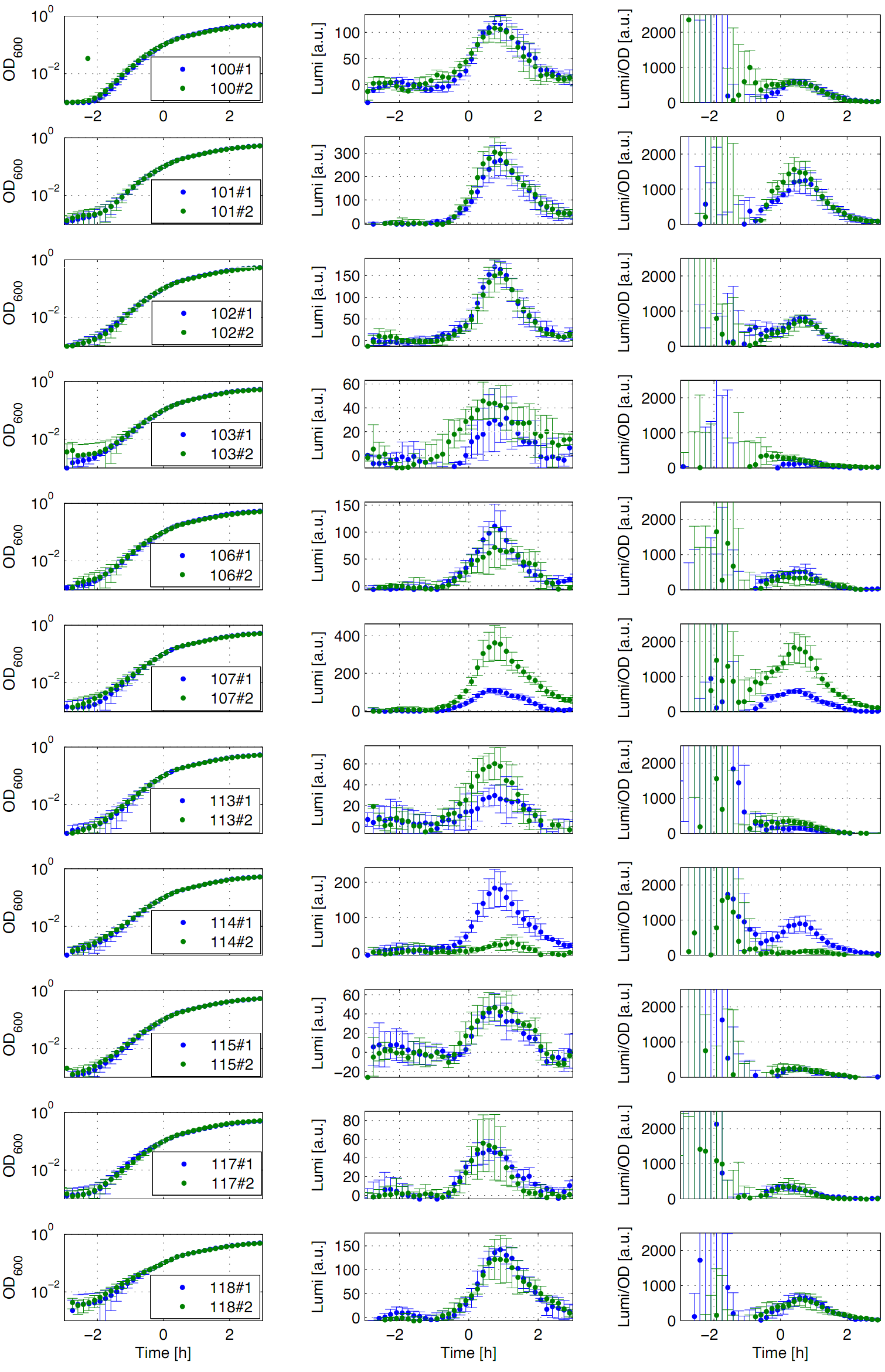
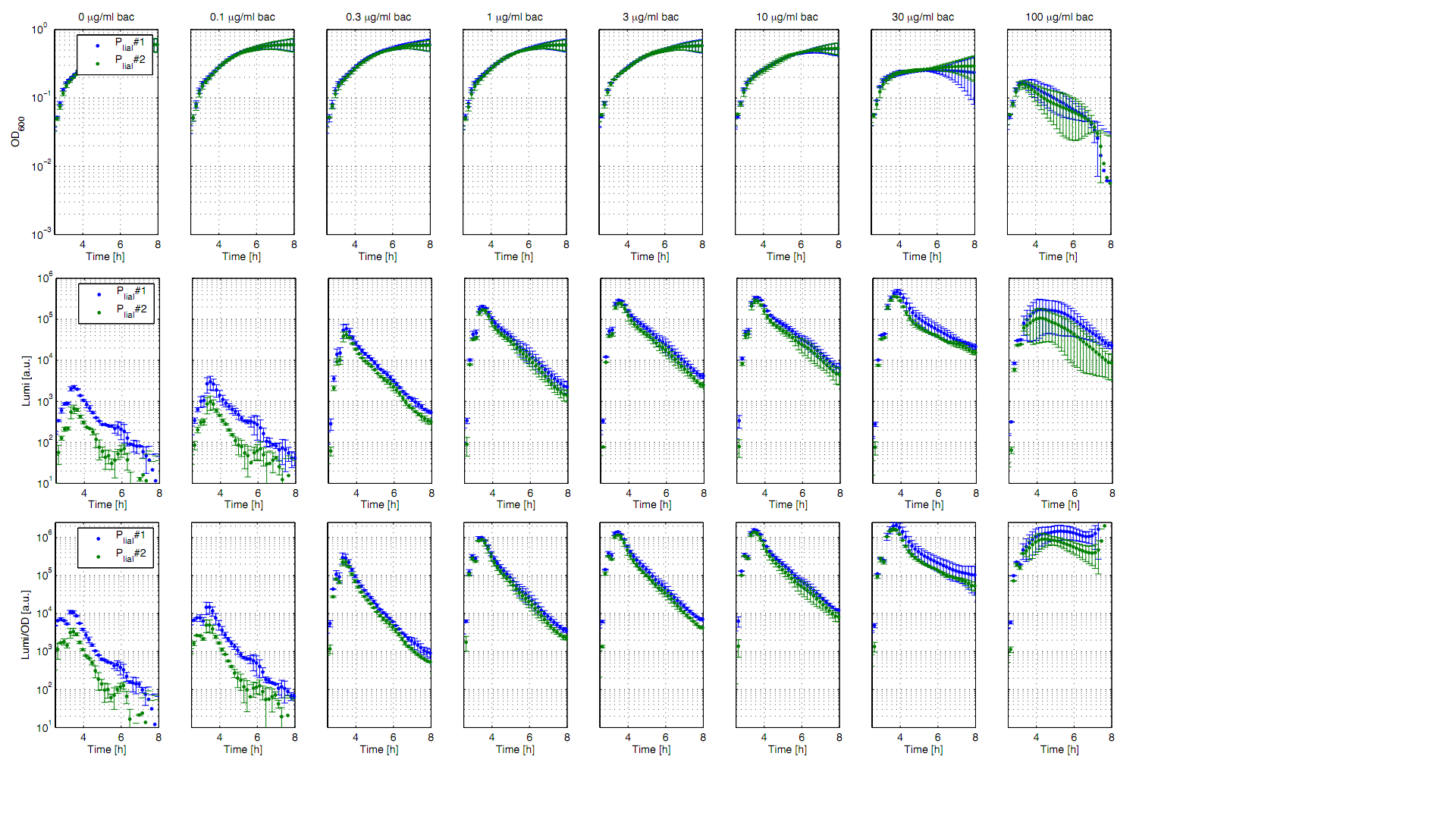
Eleven of the nineteen promoters of the [http://partsregistry.org/Part:BBa_J23100 Anderson collection] (J23100,J23101, J23102, J23103, J23106, J23107, J23113, J23114, J23115, J23117, J23118) were evaluated in the reporter vector pSBBs3C-luxABCDE from the BioBrickBox containing the lux operon as a reporter for promoter activity. The gene expression which correlates to the promoter activity leads to the expression of the lux operon with the luciferase. The luminescence which is produced by the luciferase can be measured with the plate reader (BioTek). Data derive from three undependant measurements (Fig. 1). Curves were fitted over each other (t=0, OD600=0,3) and smoothed by taking average of three neighboring values. OD600 values shown are plate reader units and about one third of the usual OD600 values. All clones show a usual growth curves. The activity of the promoters raises during the pass from the transition to the stationary phase. This maximum (t=1h) reaches from 200Lumi/OD600 (promoter J23115) to a maximum of 1500 Lumi/OD600 for the strongest promoter (J23101). Afterwards the activity goes down to the beginning level (t=2h). The oscillation of luminescence (Lumi/ OD600 in the beginning of the curves are due to the small OD600 and do not mean a high promoter activity. The luminescence of one clone of the promoters J23107 and J23114 do not show activity where in future a second clone with promoter activity should be measured. In comparison to all the other evaluated Bacillus promoters these Anderson promoters showed a very low acitivity in B. subtilis.
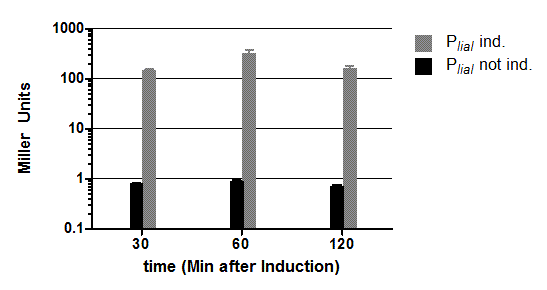
To measure the activity not only with the lux reporter operon, four promoters of the Anderson collection were cloned into the reporter vector pSBBs1C-lacZ to do β galactosidase assays and then to compare the results of the strength of these promoters in B. subtilis. (Fig. 2)
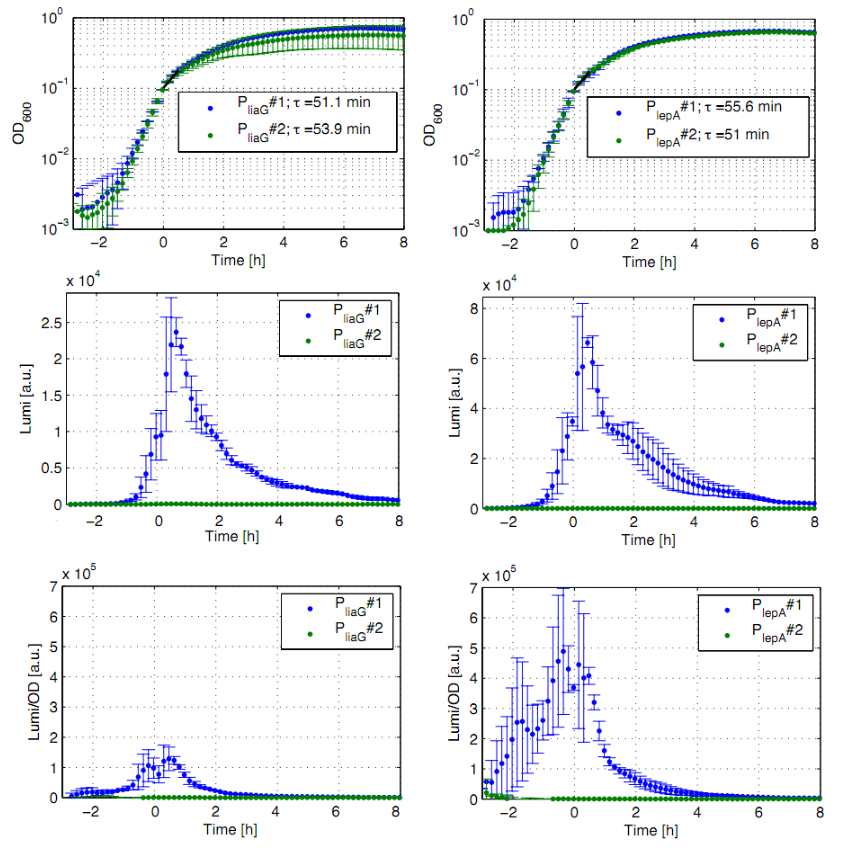

The β galactosidase assay of the constitutive Bacillus promoters Pveg and PliaG was repeated three times. Data show one representative result. Two undependant clones of B. subtilis with the same construct were measured and their mean with standard deviation is show in the graph. In the beginning of the growth curve both promoters show a small activity. But then it raises to a maximum before it decreases to the begininng level after about seven hours (Data not shown). Summing up the course of activity of both promoters Pveg and PliaG is very similar based on the growth curve. The highest beta galactosidase activity and therefore the highest activity of the promoter Pveg can with an maximum of 65 Miller units be found during the transfer from the logarithmic to the stationary phase. This is about five times higher than the acitivity of the promoter PliaG with an maximum activity of about 12 Miller Units.
Sporobeads
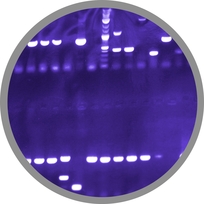
To purify our spores from the vegetative cells we treated the samples, that were grown for 24 hours in DS-Medium, with three different methods. The data is summarized in the following table:
| all cells | mature spores | immature spores | |
|---|---|---|---|
| untreated wildtype | 7.29 x 108 /ml | 1 x 108 /ml 13.71% | 0.04 x 108 /ml 0.55% |
| untreated PcotYZ-cotZre-gfp-terminator | 6.79 x 108 /ml | 1 x 108 /ml 14.72% | 0.13 x 108 /ml 1.9% |
| French Press wildtype | 4.87 x 108 /ml | 2.1 x 108 /ml 43% | 0.05 x 108 /ml 1% |
| French Press PcotYZ-cotZre-gfp-terminator | 4.75 x 108 /ml | 1.88 x 108 /ml 39.58% | 0.05 x 108 /ml 1% |
| Sonification wildtype | 4.6 x 108 /ml | 1.22 x 108 /ml 26.52% | 0.1 x 108 /ml 2% |
| Sonification PcotYZ-cotZre-gfp-terminator | 6.72 x 108 /ml | 1.53 x 108 /ml 22.77% | 0.23 x 108 /ml 3% |
| Lysozyme wildtype | 2.48 x 108 /ml | 1.58 x 108 /ml 63.7% | 0 /ml |
| Lysozyme PcotYZ-cotZre-gfp-terminator | 1.05 x 108 /ml | 1.05 x 108/ml 100% | 0 /ml |
Tab. 1 shows that after treatment with French Press and ultrasound the number of spores compared to the untreated samples was increased. We assume the reason for this was the impurity of these samples that derived from damaged vegetative cells. Thus, during counting it was not always possible to distinguish between mature spores and cell waste. However, a huge difference in number of vegetative cells and spores between untreated and lysozyme treated samples was noticeable under microscopy as it is visualized in the table above and the pictures below. Because the vegetative cells were lyzed and not damaged it was easy to recognize the mature spores.
Germination Stop
We induced our germination-mutant strains to sporulate in Difco sporulation media. Then we measured the germination rate of mutant spores in a germination assay.

Strains:
B40 -- cwlD::kan, sleB::mls, cwlJ::spec
B41 -- cwlD::kan, sleB::mls, gerD::cat
B42 -- cwlD::kan, cwlJ::spec, gerD::cat
B43 -- gerD::cat, sleB::mls, cwlJ::spec
B46 -- cwlD::kan, cwlJ::spec, gerD::cat, sleB::mls
B47 -- gerD::cat, sleb::mls, cwlJ::spec, cwlD::kan
The plate growth demonstrates the inability of our mutant spores to germinate. We can say that fewer than 1 out of 3x10^7 spores of strains B40, B41, B43, B46, and B47 germinated.
 "
"