Team:Cambridge/Safety
From 2012.igem.org
CharlotteBG (Talk | contribs) |
CharlotteBG (Talk | contribs) |
||
| Line 17: | Line 17: | ||
Several techniques used in the course of this project call for the use of potentially hazardous substances which range from causing minor skin irritations to being toxic if ingested. For this reason we have created risk assessment pages for each of the protocols used in the course of our research. These are not intended to be used instead of departmental safety procedures, but outline the major concerns for each protocol for reference by future teams. MSDS sheets for reagents used in the course of our project are also available on this wiki. | Several techniques used in the course of this project call for the use of potentially hazardous substances which range from causing minor skin irritations to being toxic if ingested. For this reason we have created risk assessment pages for each of the protocols used in the course of our research. These are not intended to be used instead of departmental safety procedures, but outline the major concerns for each protocol for reference by future teams. MSDS sheets for reagents used in the course of our project are also available on this wiki. | ||
| - | Sodium Fluoride is hazardous and was necessary for testing our fluoride riboswitch. Full departmental risk assessments were completed before working with this substance and a brief risk assessment outlining the main dangers can be found on this wiki ([[Team:Cambridge/RiskAssessments/ | + | Sodium Fluoride is hazardous and was necessary for testing our fluoride riboswitch. Full departmental risk assessments were completed before working with this substance and a brief risk assessment outlining the main dangers can be found on this wiki ([[Team:Cambridge/RiskAssessments/NaF|here]]) as well as a MSDS. |
We anticipate that elements of our project could be used for a wide variety of purposes and these may include the use of potentially harmful substances. Any future teams would need to consider the safety implications of their project on a case by case basis, though our project should not raise any safety concerns. | We anticipate that elements of our project could be used for a wide variety of purposes and these may include the use of potentially harmful substances. Any future teams would need to consider the safety implications of their project on a case by case basis, though our project should not raise any safety concerns. | ||
Revision as of 12:33, 14 August 2012
Safety
N.B. This page is a work in process and will be added to and improved over the course of the project.
iGEM safety questions
1. Would any of your project ideas raise safety issues in terms of:
- researcher safety,
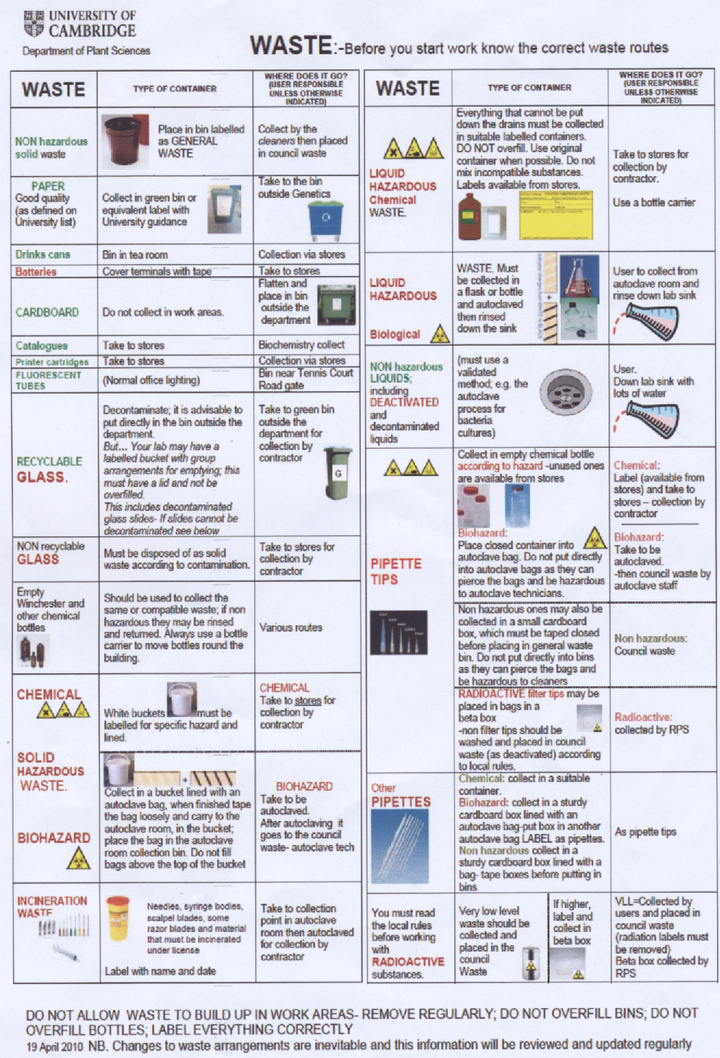
Our laboratory is only authorised to use Biosafety level 1 (non-pathogenic) bacteria and we therefore consider our project to be harmless to researchers as none of our experiments have any known action in increasing the pathogenicity of the bacteria we use or the host range. It is departmental policy however to treat all bacteria as potential pathogens and to take suitable safety precautions. We understand that it is essential to wear suitable PPE (personal protective equipment) at all times, in our experiments this usually means labcoats and gloves. All laboratory equipment and bacterial cultures are to be decontaminated by autoclaving. A copy of the departmental regulations for waste disposal is available at the bottom of this page.
Several techniques used in the course of this project call for the use of potentially hazardous substances which range from causing minor skin irritations to being toxic if ingested. For this reason we have created risk assessment pages for each of the protocols used in the course of our research. These are not intended to be used instead of departmental safety procedures, but outline the major concerns for each protocol for reference by future teams. MSDS sheets for reagents used in the course of our project are also available on this wiki.
Sodium Fluoride is hazardous and was necessary for testing our fluoride riboswitch. Full departmental risk assessments were completed before working with this substance and a brief risk assessment outlining the main dangers can be found on this wiki (here) as well as a MSDS.
We anticipate that elements of our project could be used for a wide variety of purposes and these may include the use of potentially harmful substances. Any future teams would need to consider the safety implications of their project on a case by case basis, though our project should not raise any safety concerns.
- public safety,
We understand public apprehension surrounding the use of genetically modified bacteria, in the unlikely event that members of the public came into contact with the bacteria used in our project we anticipate that there would be no threat to public safety as we are using non-pathogenic strains.
- environmental safety?
While we appreciate that pathogenic strains of the species we are using exist, we are using disabled, non-pathogenic strains that should otherwise interact in an identical manner with the environment. We therefore anticipate that any released bacteria would be disadvantaged and are not expected to survive outside of the favorable conditions engineered in the lab.
2. Do any of the new BioBrick parts (or devices) that you made this year raise any safety issues? If yes,
- did you document these issues in the Registery?
- how did you manage to handle the safety issue?
- how could other teams learn from your experience
Our biobricks are designed to create a biological system whereby a bacterial cell takes in metal ions and this induces a light output. None of our biobricks increase either the pathogenicity of the bacteria used or the range of usable hosts.
Our biobricks do not raise any safety issues.
3. Is there a local biosafety group, committee or review board at your institution?
- If yes, what does your local biosafety group think about your project?
Our project and protocols have been reviewed and accepted by our advisers and the departmental safety officer as suitable.
- If no, which specific biosafety rules or guidelines do you have to consider in your country
4. Do you have any other ideas how to deal with safety issues that could be useful for future iGEM competitions? How could parts, devices and systems be made even safer through biosafety engineering?
Additional safety information
- Risk Assessments Risk assessments for any experiments we do and safety information for carrying out any of the protocols listed on this wiki.
- MSDS Sheets Materials Safety Data Sheets for the reagents used in the course of our project.
- Protocols Protocols used in the course of our project.
- Waste procedure summary A copy of several waste safety posters around the department:
 "
"