Self-service!
To mark a part as finished, add "✓" before the title. To mark it as taken, add your name(s) after the title in the following way: "<title> - <name(s)>".
For TAs
- Buy antibodies! [http://www.abcam.com/VP16-tag-antibody-ab4809.html]
LovTAP
Original RO plasmid
✓ RO plasmid + Spc transfromation
Using our competent cells. Done on June the 28th, and on June the 29th. Plates with colonies in the fridge.
✓ Miniprep and gel
Done on June the 29th and July 4th. Looks like transformation was a success!!
✓ Maxiprep
Done the 12th July. Digestion and gel done the 16th July.
Original LovTAP plasmid
Mini-preps done and correct plasmids confirmed.
✓ Transformation LovTAP + Amp
Done the 29th June. One plate in the fridge, dated 28-06-12.
✓ Maxiprep
Done the 12th July. Digestion and gel done the 16th July.
Make selection possible
✓ Design primers to integrate puromycin into the LovTAP plasmid - Sander
Not done yet. Still need to decide in which direction the integration should be done (integrate puromycin into the LovTAP plasmid or integrate LovTAP into pMP-PB).
- Puromycin → LovTAP plasmid
- No control over directionality (only a single available RS)
- Less worries about mutations (mutated versions of puromycin will just die during the selection process)
- LovTAP → pMP-PB
- Integration easy and controlled (lots of easy to use RS)
- (Too?) Efficient expression thanks to strong promoter & intron.
- Interference of non-used gene sequences?
Insert puromycin:
Run the following primers on pMB-PB (4 random bases, PsiI, matching part):
Primer 1 (54.9 °C, 10 + 16bp): 5' - CTGC TTATAA GTCTGACGCTCAGTGG → 3' Primer 2 (55.5 °C, 10 + 14bp): 5' - GCTT TTATAA CTTCATCCCCGTGG → 3' Annealing temp: 54.9 °C
Digestion: NEBuffer 4 & PsiI @ 37°C
Check if the puromycin was inserted in the correct direction (if there is product, the puromycin was inserted in the correct direction):
Primer 1 (55.3 °C, 31bp): 5' - CTAAATTGTAAGCGTTAATATTTT → 3' Primer 2 (53.5 °C, 13bp): 5' - CCGACGTCGAGGT → 3' Annealing temp: 53.5 °C
✓ Design primers to integrate puromycin into the RO plasmid - Sander
See LovTAP plasmid design, uses same primers.
✓ Design primers to integrate LovTAP into pMP-PB - Sander
Primer 1: SpeI Primer 2: XbaI Primer 1 (57.6 °C, 10 + 12bp): 5' - GATA ACTAGT CCTAGGCGCGCC → 3' Primer 2 (57 °C, 10 + 22bp): 5' - AGTA TCTAGA TTAATTAAGAGGTACCGGAATT → 3' Annealing temp: 57 °C
✓ Design primers to integrate RO into pMP-PB - Sander
Primer 1: AscI Primer 2: XbaI Primer 1 (62 °C, 12 + 14bp): 5' - ACCA GGCGCGCC CCGCATCTCGAGCG → 3' Primer 2 (62 °C, 10 + 17bp): 5' - CTAT TCTAGA CAGGTTTCCCGACTGGA → 3' Annealing temp: 62 °C
Investigate use of different antibiotics in the plasmids
In general using the same antibiotics will result in a 50/50 split of intake of the plasmid. Alternatives are:
- Puromycin
- Zeocin
- Blasticidin
- Hygromycin B
Where Zeocin has a significantly different method of operation (Zeocin cuts up DNA while the other antibiotics interfere with the protein synthesis process).
Produce the modified plasmids
Using the previously designed primers:
- PCR → part we want to add surrounded by RS
- Digestion of the plasmid & PCR product
- Ligation
- Selection
Transfect
Transient transfection -Alex, Mouna, June
Can be done as soon as the plasmid has been produced in high quantities (maxi-prep). Maxiprep of both RO and LovTAP done the 12th July. LB medium prepared the 16th July.
Stable transfection
Need to modify the plasmids for selection & perform max-prep.
Experiment
Basic tests
Test whether the LovTAP works (just test whether something is being expressed). Then check if there is a difference in expression level between the dark state and the light state. This difference should be a factor of about 5 (not sure, need to check again?).
Other tests
Try to optimize expression (amount of light, plasmid proportions, etc...).
Increase efficiency
The version of LovTAP we're using isn't the ultimate one, if we have enough time (and most of all, got the thing working in mammalian cells) we could try some form of site-directed mutagenesis to increase the efficiency.
Fussenegger
Nothing done yet! Come on, lots of work to do!
Get the main plasmid DNA sequence
Waiting for Fussenegger...
Create the RO plasmid
We have the backbone, just need to clone in the RO system -> design primers
Transfect into CHO
Experiment with HEK
Bioreactor
We talked to Dominique, who proposed a rather easy solution. Take an incubator and cover the window, add LEDs to a tubespin holder for light-state experiments and use a dark box (fixed to the shaker) for the dark-state experiments.
Requisition and modifiy an incubator
Get exclusive access to an incubator and modify it to not let any light pass in (stick something on the front window...).
Create LED strips
We need ~8 candella per tube (~8 lumen) of blue light. The LovTAP experiment used light at 468nm. The LEDs need to be ordered and the light chain+circuitry+power supply needs to be created.
Buy 20 of the following (2 packets): [http://www.ledstar.ch/10-Stueck-LowCost-LED-5mm-blau-13000mcd-ultrahell-unsortiert Blue LEDs]
Calculate the right resistances (and buy them). If LEDs connected in parallel groups where each group is composed of 2 LEDs in parallel for a resistance (see drawing if not clear) → 65 ohm ("rounded" up to 68 ohm to be able to buy them) (http://www.ledstar.ch/Widerstand-025Watt-68-Ohm_1)
Create the circuit (use a few AA batteries?). 6V (can be supplied by either four AA batteries or by a relatively standard charger.
Thought 2: Use 12V as there are more power sources for it?
The batteries will only last 6-9h under full load, so rechargeable batteries or external chargers need to be considered.
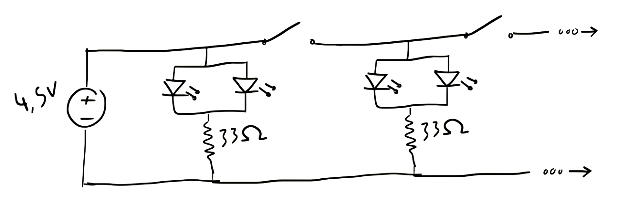
It can also be done more efficiently with 3 AA batteries → 4.5V (not all switches are necessary):
Buy LED sticks? - Diego
Dasgip makes them. See July the 13th.
Create a "dark box"
Modify the plexiglass box in the corner of the cell lab so it blocks all light (add paper/aluminium foil or paint). This is not needed for the first experiments where a bit of tape should suffice.
Build lighting model
3D print bioreactor?
Small scale metallic
Biobricking
Nothing done yet! Use the primer design helper (in the dropbox primers folder) to quickly design the primers.
Make a list of all biobrickable stuff!
- TNFR:Fc
- LovTAP mammalian
- Melanopsin
Wiki
Made a basic templating system that can be extended later (shouldn't change the code of the pages themselves).
Start the designing! - Sander
Decided on the (slightly kitschy) lab-bench design. Comparable to the style of the 2010 EPFL iGEM team, but with more doodling and randomly placed objects in the background!
Fill it!
The templates should be complete enough to be able to work with the wiki now without needing to reenter all data later. In progress!
Others
✓ Competent cell preparation
Requisite for E. coli transformation. Done the 25th of May. The cells are in the -80ºC freezer.
Safety
All members followed safety course given by university. Need to meet with the [http://people.epfl.ch/cgi-bin/people?id=163673&lang=en Stéphane Karlen] to get a more detailed (more relevant) safety opinion. Email sent asking for an appointment (12-7 Diego).
 "
"