Team:Queens Canada/ChimeriQ/Results
From 2012.igem.org
(Difference between revisions)
| Line 64: | Line 64: | ||
<div id="contentbox" style="border-bottom:none;"> | <div id="contentbox" style="border-bottom:none;"> | ||
Our work the Cohesin-Dockerin system had the ultimate goal of being able to create a chimeric flagella with a Dockerin insert, which would be able to bind with a specific Cohesin domain secreted by the bacteria. Unfortunately we were not able to reach this point, but were were able to create a part with type II cohesin from Clostridium Thermocellum as a chimeric insertion into flagellin (<a href="http://partsregistry.org/wiki/index.php?title=Part:BBa_K781003"> K781003 </a>). | Our work the Cohesin-Dockerin system had the ultimate goal of being able to create a chimeric flagella with a Dockerin insert, which would be able to bind with a specific Cohesin domain secreted by the bacteria. Unfortunately we were not able to reach this point, but were were able to create a part with type II cohesin from Clostridium Thermocellum as a chimeric insertion into flagellin (<a href="http://partsregistry.org/wiki/index.php?title=Part:BBa_K781003"> K781003 </a>). | ||
| - | <div class="thumb tleft"><div class="thumbinner" style="width:502px; margin-left: 219px;"><a href="/Image:Cohesin_mot.png" class="image" title="Fig. 1 The motility assay for the K781003 construct compared against controls expressing no flagellin construct and a non-chimeric flagellin construct. Inoculations made on 0.25% swimming agar."><img alt="Fig. 1 The motility assay for the K781003 construct compared against controls expressing no flagellin construct and a non-chimeric flagellin construct. Inoculations made on 0.25% swimming agar." src="https://static.igem.org/mediawiki/2012/7/7a/Cohesin_mot.png" width="500" height="375" border="0" class="thumbimage" /></a> <div class="thumbcaption"><div class="magnify"><a href="/Image:Cohesin_mot.png" class="internal" title="Enlarge"><img src="/wiki/skins/common/images/magnify-clip.png" width="15" height="11" alt="" /></a></div><b>Fig. | + | <div class="thumb tleft"><div class="thumbinner" style="width:502px; margin-left: 219px;"><a href="/Image:Cohesin_mot.png" class="image" title="Fig. 1 The motility assay for the K781003 construct compared against controls expressing no flagellin construct and a non-chimeric flagellin construct. Inoculations made on 0.25% swimming agar."><img alt="Fig. 1 The motility assay for the K781003 construct compared against controls expressing no flagellin construct and a non-chimeric flagellin construct. Inoculations made on 0.25% swimming agar." src="https://static.igem.org/mediawiki/2012/7/7a/Cohesin_mot.png" width="500" height="375" border="0" class="thumbimage" /></a> <div class="thumbcaption"><div class="magnify"><a href="/Image:Cohesin_mot.png" class="internal" title="Enlarge"><img src="/wiki/skins/common/images/magnify-clip.png" width="15" height="11" alt="" /></a></div><b>Fig. 6</b> The motility assay for the K781003 construct compared against controls expressing no flagellin construct and a non-chimeric flagellin construct. Inoculations made on 0.25% swimming agar.</div></div></div> |
<div id="contentbox" style="color:white; clear:both; border-bottom:none;"> | <div id="contentbox" style="color:white; clear:both; border-bottom:none;"> | ||
fas | fas | ||
Revision as of 03:28, 27 October 2012
Control
ChimeriQ - Results
Overview
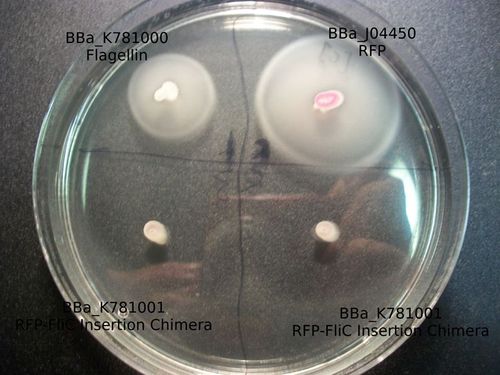
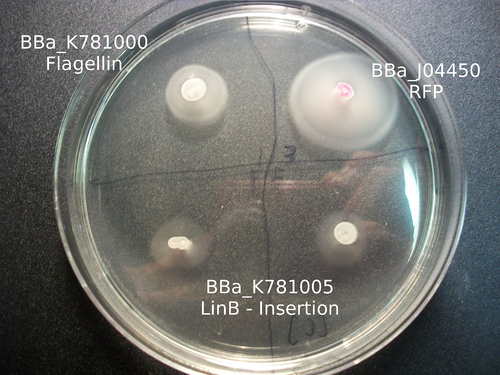
As the goal of our project was to create chimeric flagella, the majority of our results were based around the creation of flagella with various inserts. In order to do so, we needed to make a mechanism by which we could easily create this chimeric primers. In doing so, we were able to create the part K781000 . This is the complete flagellin coding sequence with biobrick standard cut sites removed, under the promoter R0010 and RBS B0034. This part can be used to over express the flagellin monomer. PCR overlap extension will make an insertion at the annotated site, given that the insert is in the appropriate format. The resulting chimera will express protein immediately after the PCR reaction is transformed.
Fluorescence
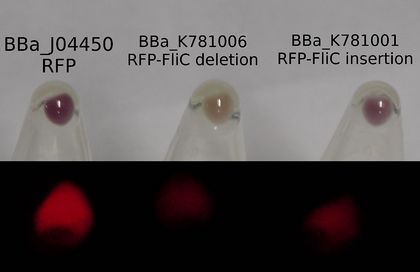
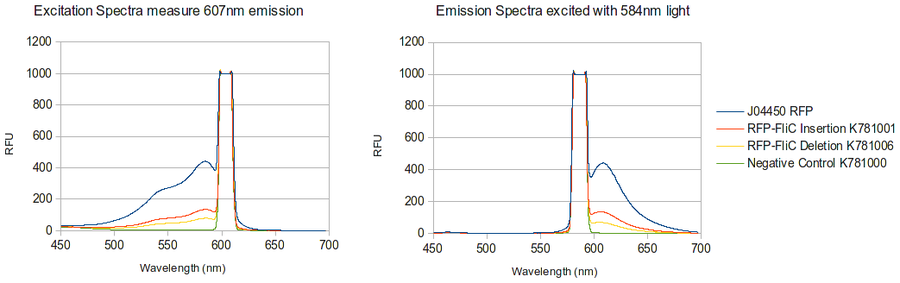
The majority of our results fell under this section of our work as we were able to create both the RFP-FliC Insertion and deletion chimeras. These parts were successfully characterized and determined to show expression of the red fluorescent protein from J04500. As can be seen below, there appears to be less detectable fluorescence in cell cultures expressing the different types of chimeras. This result can be expected for a number of possible reasons.
Catalysis
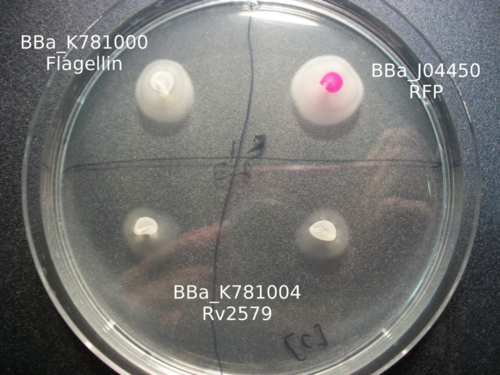
Our work with catalysis mainly involved working with three enzymes: LinB, Rv2579, and XylE. Using K781000, we were able to create three parts: BBa_K781005 , BBa_K781004 , and BBa_K781007 . Based on the images below, it appears as if we were able to incorporate these enzymes into the bacteria, but more research much be done in order to characterize the enzyme activity.
The Cohesin-Dockerin System
Our work the Cohesin-Dockerin system had the ultimate goal of being able to create a chimeric flagella with a Dockerin insert, which would be able to bind with a specific Cohesin domain secreted by the bacteria. Unfortunately we were not able to reach this point, but were were able to create a part with type II cohesin from Clostridium Thermocellum as a chimeric insertion into flagellin ( K781003 ).
fas
 "
"