Team:HokkaidoU Japan/Notebook/aggregation Week 2
From 2012.igem.org
(→Transformation) |
(→Digestion) |
||
| Line 418: | Line 418: | ||
[[image:HokkaidoU2012 120714 pT7-RBS-Ag43-dT on pSB1C3 digeation EP .jpg|thumb|Digestion results]] | [[image:HokkaidoU2012 120714 pT7-RBS-Ag43-dT on pSB1C3 digeation EP .jpg|thumb|Digestion results]] | ||
| + | <br style="line-height: 0; clear: both;" /> | ||
</div></div> | </div></div> | ||
<div class="hokkaidou-notebook-daily"> | <div class="hokkaidou-notebook-daily"> | ||
| + | |||
===July 14th=== | ===July 14th=== | ||
<div class="hokkaidou-section"> | <div class="hokkaidou-section"> | ||
Revision as of 01:51, 27 September 2012
Contents |
July 9th
pT7 + RBS (3A Assembly) and Ag43 + dT (standard assembly) of ligation products were transformed and incubated. We confirmed ideal insert DNA (pT7 and RBS or Ag43 and dT) were inserted by colony PCR.
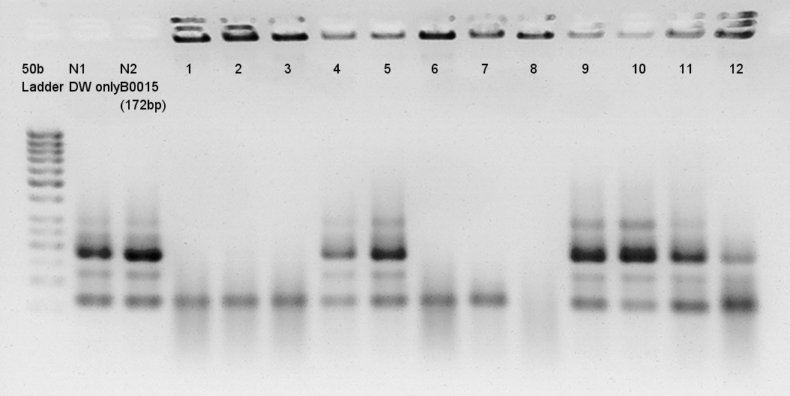
Colony PCR
Colony PCR for assembly products.
- Picked up colony from LB plates by Autoclaved toothpicks.
- Re-suspension into 10 ul DW in 1.5 ml eppendorf tubes.
- 4 ul was added in PCR reaction solution, and 6 ul was mixed with 200 ul LB.
- Ran PCR machine in recipe below.
PCR reaction solution
| DNA solution | 4 ul |
| KapaTaq ready mix | 5 ul |
| BioBrick prefix forward primer | 0.5 ul |
| BioBrick suffix reverse primer | 0.5 ul |
| Total | 10 ul |
PCR recipe
(pT7 + RBS)
| Number | Degree | Second |
| 1 | 94 | 120 |
| 2 | 94 | 30 |
| 3 | 68 | 60 |
| 4 | 4 | HOLD |
Cycle:2~3 x 40
(Ag43 + dT)
Ag43 + dT (+ Biobrick prefis & suffix) is about 3290bp.
| Number | Degree | Second |
| 1 | 94 | 120 |
| 2 | 94 | 30 |
| 3 | 68 | 180 |
| 4 | 4 | HOLD |
Cycle:2~3 x 35
Electrophoresis results
Electrophoresis for (pT7 + RBS) by 2% agarose gel and (Ag43 + dT) by 1% agarose gel.
pT7 + RBS on pSB1K3
bbp-Insert-bbs:86bp
Ag43 + dT on pSB1AK3 bbp-Insert-bbs:3290bp
We couldn't confirm insert DNA were really ligated with Vector or not.
Next step, we tried confirmation of insert DNA by electrophoresis of extracted plasmids.
For plasmid extraction, we needed to incubate in liquid culture.
Incubate for plasmid extraction
- Prepared 1800 ul LB solutions.
- Mixed 200 ul of cultures (suspention were incubated for 2 hrs) and antibiotics(Km (pT7-rbs on pSB1K3), Amp(Ag43-dT on pSB1AK3)).
- Incubated for 15 hrs and 30 min.
July 10th
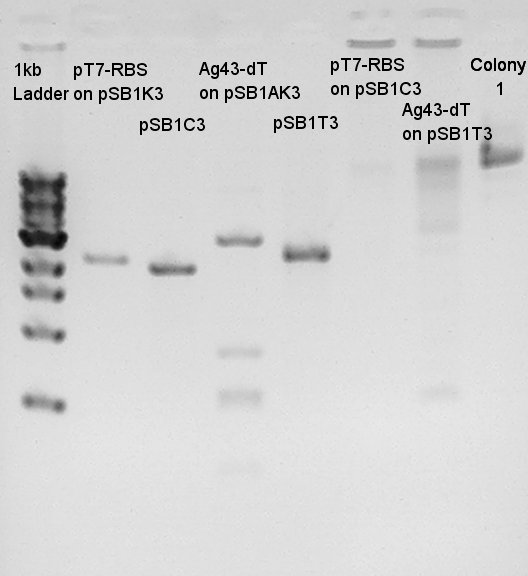
Plasmid extraction
Extracted plasmids from some colonies (we selected colonies which showed more correct bands than other colonies, pT7+RBS were No. 4, 5, 9, 10 colonies and Ag43 + dT were No. 1, 2, 3, 4 colonies)incubated in LBA(Ag43 + dT) and LBK(pT7 + RBS) for 15 hrs and 30 min.
- Extracted from LB culturing products by using FastGene Plasmid Mini kit(Nippon Genetics).
- Electrophoresis (Used pre-migrated 1% agarose gels with 5 ul EtBr)in 30 min.
pT7 + RBS on pSB1K3(Total 2247bp)
Ag43 + dT on pSB1AK3(Total 6444bp)
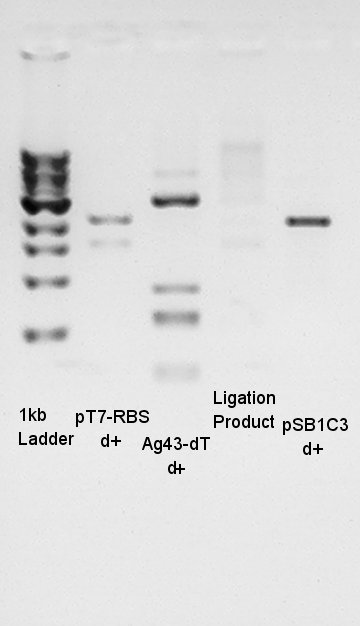
To confirm more correctly about insert DNA, we tried to digest these DNA with EcoRI and PstI.
Digestion
Digestion for confirmation of insert DNA. Each exracted DNA were digested with E & P.
Digestion recipe
pT7-RBS
| pT7-RBS | 1.5 ul |
| EcoRI | 1 ul |
| PstI | 1 ul |
| 10xH buffer | 2 ul |
| DW | 14.5 ul |
| Total | 20 ul |
Digestion recipe
Ag43-dT
| Ag43-dT | 4 ul |
| EcoRI | 1 ul |
| PstI | 1 ul |
| 10xH buffer | 2 ul |
| DW | 12 ul |
| Total | 20 ul |
Digestioned at 37c for 2 hrs.
Insert DNA were correct we thought. We tried digestion for 3A Assembly[pT7-RBS+Ag43-dT+pSB1C3]
Digestion
Digestion for 3A Assembly.
pT7-RBS
| DNA | 17 ul |
| EcoRI | 1 ul |
| SpeI | 1 ul |
| 10xH buffer | 3 ul |
| DW | 8 ul |
| Total | 30 ul |
Ag43-dT
| DNA | 12.5 ul |
| XbaI | 1 ul |
| PstI | 1 ul |
| 10xH buffer | 2 ul |
| DW | 3.5 ul |
| Total | 20 ul |
pSB1C3
| DNA | 20 ul |
| EcoRI | 1 ul |
| PstI | 1 ul |
| 10xH buffer | 3 ul |
| DW | 5 ul |
| Total | 30 ul |
Reacted for 2 hrs at 37c.
July 11th
Ligation
Ligation of pT7-RBS + Ag43-dT + pSB1C3(3A Assembly)
Ligation recipe
| pT7-RBS | 2 ul |
| Ag43-dT | 2 ul |
| pSB1C3 | 3 ul |
| Ligation Mighty Mix(TAKARA) | 8 ul |
| Total | 16 ul |
Ligation reaction time was written below.
| Degree | Minute |
| 16 | 30 |
| 65 | 10 |
| 4 | Hold |
Transformation
Transformation for pT7-RBS + Ag43-dT + pSB1C3 into BL21(DE3)(E.coli which have T7 polymerase coading site in their genome).
- Added DNA solutions (Ligation products) 1 ul to compitent cell of BL21(DE3).
- Incubated on ice for 30 min.
- Heatshock for 1 min at 42c.
- Added 200 ul of LB to transformed BL21(DE3) solution.
- Pre-incubate for 2 hrs
- Spread 200 ul of supernant of LB&BL21(DE3) solution to LBC plate.
- 50ul of LB&BL21(DE3) solution was added to 200ul LB solution then spread 200 ul to LBC plate.
- Incubated for 23 hrs and 30 minutes.
July 12th
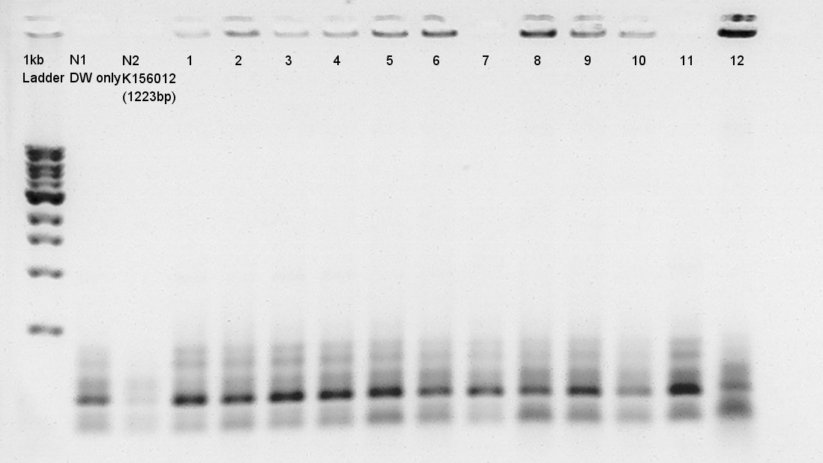
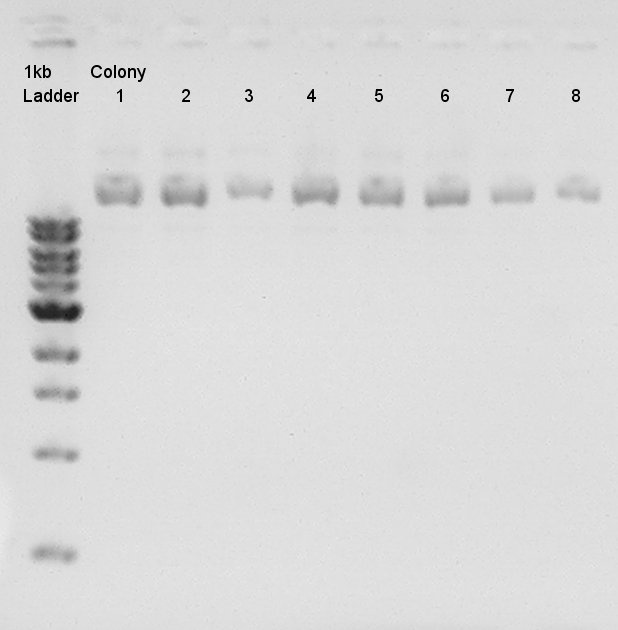
Colony PCR
Colony PCR for ligation product.Each products were reacted in recipes written below.
- Picked up each 16 colonies from LBC plates by Autoclaved toothpicks.
- Dipped into 10 ul DW in 1.5 ml eppendorf tubes.
- From 10 ul DW, 4 ul was added in PCR reaction solution (below), 6 ul was mixed with 200 ul LB.
- Ran PCR machine in recipe below.
- Electrophoresis for confirmation of PCR results.
PCR reaction solution
| DNA solution | 4 ul |
| KapaTaq ready mix | 5 ul |
| BioBrick prefix forward primer | 0.5 ul |
| BioBrick suffix reverse primer | 0.5 ul |
| Total | 10 ul |
PCR recipe
(pT7 + RBS)
| Number | Degree | Second |
| 1 | 94 | 120 |
| 2 | 94 | 30 |
| 3 | 68 | 90 |
| 4 | 4 | HOLD |
Cycle:2~3 x 40
Liquid culture
Liquid culture for some colonies used in colony PCR.
- Prepared 200 ul LB solutions.
- To these LB solutions, added 6 ul of LB solutions (colony PCR solutions were pre-incubated for 3 hrs) and added 2 ml LB and antibiotic(Cp).
- Incubated for 18 hrs and 30 min.
July 13th
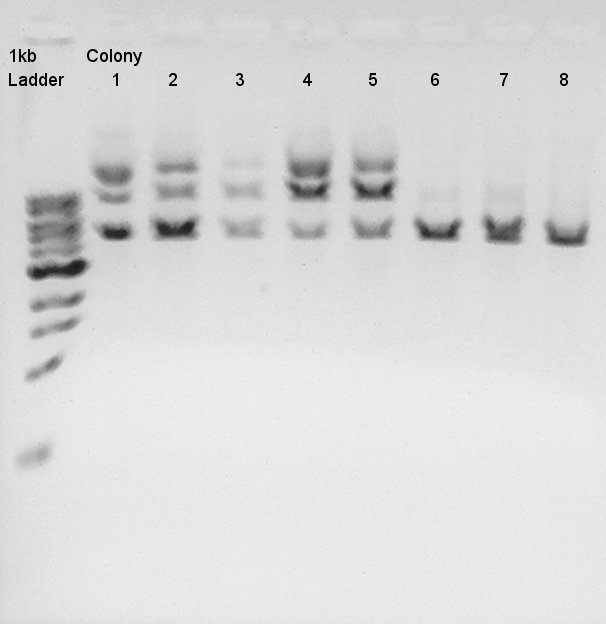
Plasmid extraction
Plasmid extraction from colony No. 1~8.
We used mini-prep kit FastGene Plasmid Mini Kit (Nippon Genetics), and got 50 ul of DNA solution.
Digestion
Digestion to confirm how DNA fragments ligated.
Digestion Recipe
| DNA | 4 ul |
| EcoRI | 0.5 ul |
| PstI | 0.5 ul |
| 10xH buffer | 2 ul |
| DW | 13 ul |
| Total | 20 ul |
July 14th
Ligation
Ligation for digestion fragments written above.
Ligation recipe
Each DNA fragments (pT7-RBS + pSB1C3 and Ag43-dT + pSB1T3) were reacted in recipe written below.
| Insert | 5 ul |
| Vector | 1 ul |
| Ligation Mighty Mix(TAKARA) | 6 ul |
| Total | 12 ul |
| Degree | Minute |
| 16 | 30 |
| 65 | 10 |
| 4 | Hold |
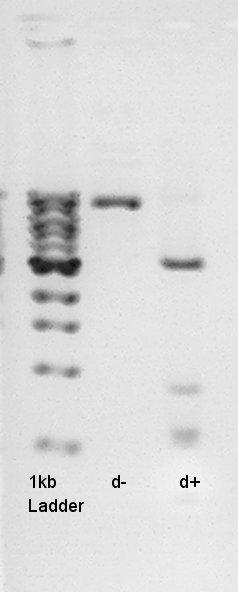
We tried electrophoresis of colony no. 1 (see colony pcr result at 9th) from ligation product.
Transformation
Transformation for ligation products written above.
- Added DNA solutions (Ligation products) 1 ul to DH5α compitent cell.
- Incubated on ice for 30 min.
- Added 600 ul of LB to transformed DH5α solution.
- Pre-incubate for 2 hrs
- Spread 300 ul of LB&DH5α solution to LBC and LBT plate, and 100 ul was added into 900 ul of LB.
- Spread 300 ul of LB&DH5α solution from 1000 ul LB (made at 5) to LBC and LBT plate.
- Incubated for 21 hrs.
And to confirm success of ligation about pT7-RBS-Ag43-dT on pSB1C3, we tried to digest this DNA with EcoRI and PstI once more time. Showed the result.
Liquid culture
Liquid culture for Ag43(BBa_K346007)
- Picked up one colony from plate done single colony isolation.
- Dipped into LBC solution.
- Incubated for 16 hrs.
July 15th
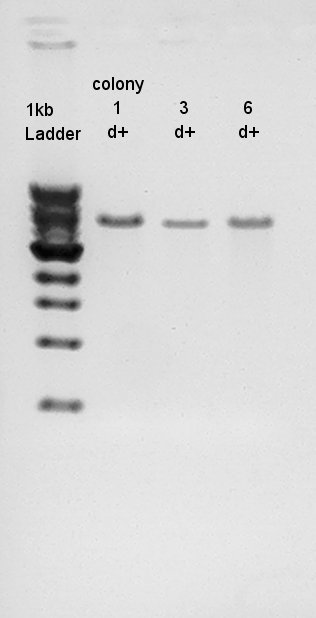
Gel extraction
Used Gel Extraction Kit (FastGene Gel/PCR extraction kit:NipponGenetics) to extract digestion products (see July 14th).
Digestion
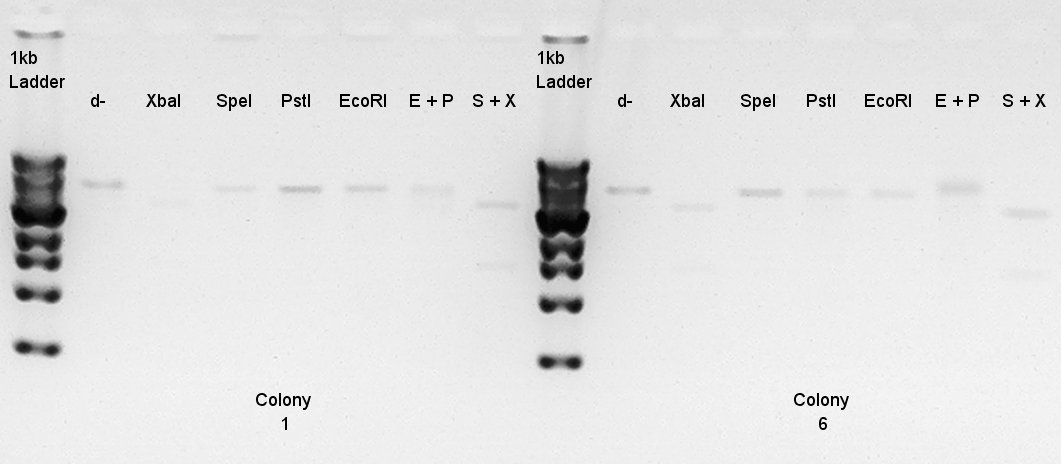
Digestion to confirm that what kind of restriction enzyme cutting sites are there in DNA (colony 1 and 6).
EcoRI
| DNA solution | 6 ul |
| EcoRI | 1 ul |
| 10xH buffer | 1 ul |
| DW | 2 ul |
| Total | 10 ul |
XbaI
| DNA solution | 6 ul |
| XbaI | 1 ul |
| 10xM buffer | 1 ul |
| BSA | 1 ul |
| DW | 1 ul |
| Total | 10 ul |
PstI
| DNA solution | 6 ul |
| PstI | 1 ul |
| 10xH buffer | 1 ul |
| DW | 2 ul |
| Total | 10 ul |
SpeI
| DNA solution | 6 ul |
| SpeI | 1 ul |
| 10xM buffer | 1 ul |
| DW | 2 ul |
| Total | 10 ul |
EcoRI + PstI
| DNA solution | 6 ul |
| EcoRI | 1 ul |
| PstI | 1 ul |
| 10xH buffer | 1 ul |
| DW | 11 ul |
| Total | 20 ul |
XbaI + SpeI
| DNA solution | 6 ul |
| XbaI | 1 ul |
| SpeI | 1 ul |
| 10xM buffer | 1 ul |
| DW | 11 ul |
| Total | 20 ul |
Ethanol precipitation
Ethanol precipitation for digestion products.
- Added 2 ul of NaoAc, 1.5 ul of glycogen and 50 ul of 100% ethanol.
- Centrifuged at 15000 rpm, 10 min at 4C.
- Removed supernatant and added 220 ul of 70% ethanol.
- Centrifuged at 15000 rpm, 5 min at 4C.
- Removed supernatant and dried out at room temperature, after that added 5 ul of DW.
Electrophoresis
Electrophoresis for digest-ethanol precipitation products.
- Added 5 ul of EtBr.
- Migrated for 30 min.
Single colony isolation
Single colony isolation for transformation products of yesterday.
- Picked up one colony from incubated LBC and LBT plate.
- Spread it on LBC and LBT plate.
- Incubated for 16 hrs.
Digestion
Digestion of Ag43(with S,P), dT(With X,P) and pT7-RBS(With E).
Ag43
| DNA solution | 9 ul |
| SpeI | 1 ul |
| PstI | 1 ul |
| 10xH buffer | 2 ul |
| DW | 7 ul |
| Total | 20 ul |
dT
| DNA solution | 1.1 ul |
| XbaI | 1 ul |
| PstI | 1 ul |
| 10xM buffer | 2 ul |
| DW | 14.9 ul |
| Total | 20 ul |
pT7-RBS
| DNA solution | 6 ul |
| EcoRI | 1 ul |
| 10xH buffer | 2 ul |
| DW | 11 ul |
| Total | 20 ul |
 "
"