Team:Colombia/Project/Experiments/Ralstonia
From 2012.igem.org
m (→Ralstonia BioBricks) |
m (→PxpsR Reporter) |
||
| Line 12: | Line 12: | ||
==PxpsR Reporter== | ==PxpsR Reporter== | ||
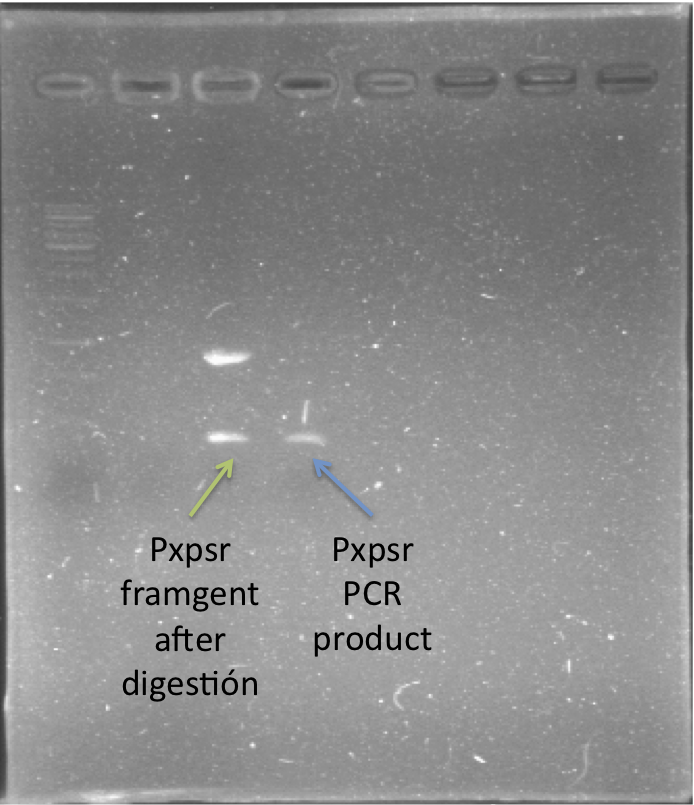
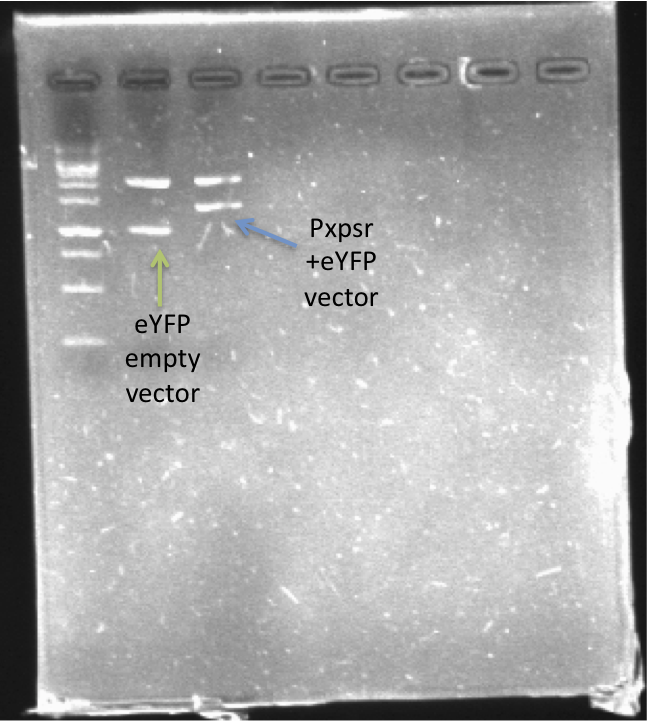
| - | In order to quantify the responsiveness of PxpsR to the presence of 3 OH-PAME we generated a PxpsR-eYFP reporter using the E0430 BioBrick (Image 2) For a more extensive and more detailed cloning procedures | + | In order to quantify the responsiveness of PxpsR to the presence of 3 OH-PAME we generated a PxpsR-eYFP reporter([http://partsregistry.org/Part:BBa_K831017 (K831017)]) using the E0430 BioBrick (Image 2). For a more extensive and more detailed explanation of the cloning procedures, please visit: [https://2012.igem.org/Team:Colombia/Notebook/Journal ''Ralstonia Journal'']. The reporter was tested in the pSB1A2 however the part was ensambled into de pSB1C3 backbone and sent to the iGEM Registry Parts. Here we show the functionality and basal activity (in absence of 3 OH-PAME) of the PxpsR-eYFP reporter (Image 3). |
[[File:PxpsR-eYFP.png|250px|thumb|center|''Image 2''. '''Confirmation of PxpsR-eYFP construct'''. After confirmation of colonies by PCR, plasmids of 2 candidates were digest with EcoRI and SpeI. Lane 1, MW (Fermentas 1KB ladder). Lane 2, Candidate 1: promotorless eYFP (E0430). Lane 3, Candidate 2: PxpsR-eYFP.''Note'': We used a reclycled gel to optimize the laboratory reagents.]] | [[File:PxpsR-eYFP.png|250px|thumb|center|''Image 2''. '''Confirmation of PxpsR-eYFP construct'''. After confirmation of colonies by PCR, plasmids of 2 candidates were digest with EcoRI and SpeI. Lane 1, MW (Fermentas 1KB ladder). Lane 2, Candidate 1: promotorless eYFP (E0430). Lane 3, Candidate 2: PxpsR-eYFP.''Note'': We used a reclycled gel to optimize the laboratory reagents.]] | ||
Revision as of 22:31, 26 September 2012
Template:Https://2012.igem.org/User:Tabima
Contents |
Ralstonia Experiments
Ralstonia BioBricks
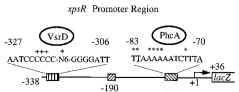
We cloned and generated a biobrick([http://partsregistry.org/Part:BBa_K831016 K831016]) for the promoter region of xpsR (PxpsR). The product of this gene is an integrator signal that regulates the expression of several virulence factors in Ralstonia solanacearum(). One of the signals that XpsR integrates is phcA which is involved in the Quorum Sensing mediated by 3 OH-PAME exclusive of R. solanacearum. We designed primers to amplify the upstream region of xpsR gene including the two binding boxes of phcA, the -35 and -10 conserved boxes and excluding the native RBS (Figure 1). This part was confirmed by sequencing. In addition, we cloned the three genes (phcA, phcS and phcR) involved in the sensing of the 3 OH-PAME(), further confirmation of these parts is still needed. Image 1 shows the enzymatic confirmation for PxpsR in the backbone pBS1C3. For a more extensive and more detailed description of the cloning procedures, please visit: Ralstonia Journal
PxpsR Reporter
In order to quantify the responsiveness of PxpsR to the presence of 3 OH-PAME we generated a PxpsR-eYFP reporter([http://partsregistry.org/Part:BBa_K831017 (K831017)]) using the E0430 BioBrick (Image 2). For a more extensive and more detailed explanation of the cloning procedures, please visit: Ralstonia Journal. The reporter was tested in the pSB1A2 however the part was ensambled into de pSB1C3 backbone and sent to the iGEM Registry Parts. Here we show the functionality and basal activity (in absence of 3 OH-PAME) of the PxpsR-eYFP reporter (Image 3).
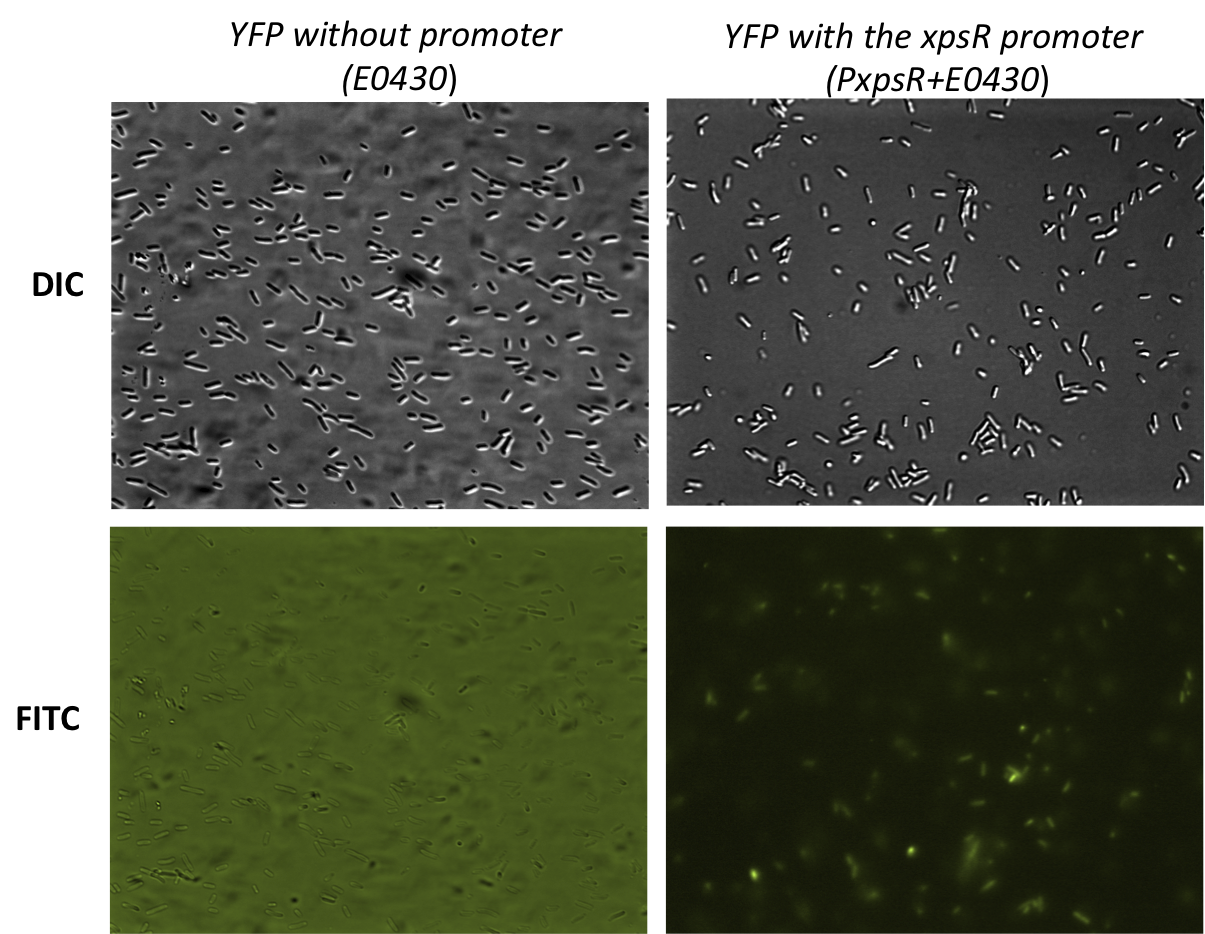
Response of PxpsR to synthetic 3 OH-PAME
Objective To determine the induced response of PxpsR in a mute Ralstonia solanacearum with the presence of synthetic 3 OH-PAME at different concentrations, using eYFP as a fluorescent reporter.
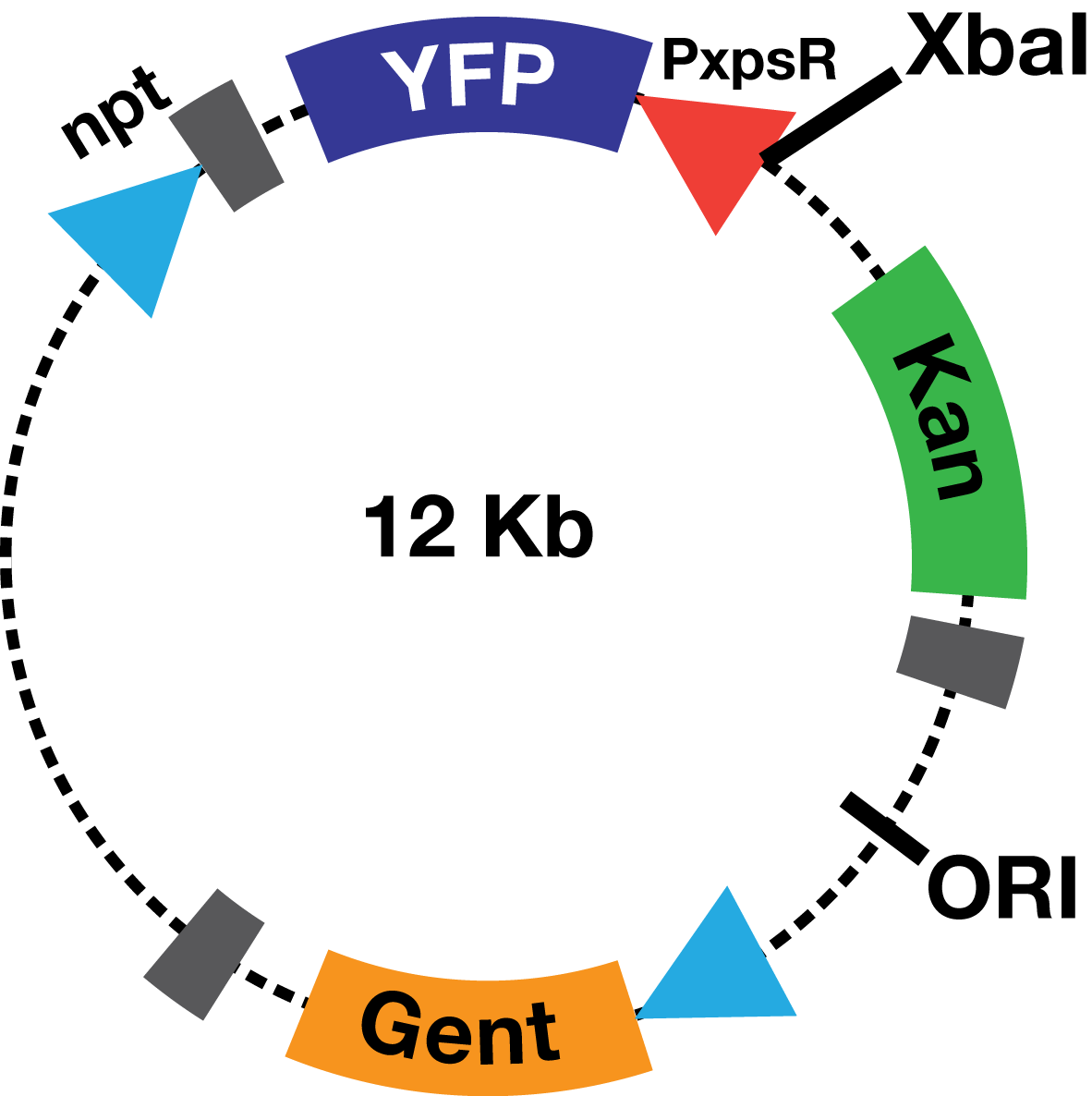
Description The PxpsR-eYFP biobrick was digested with XbaI and SpeI, and then was ligated to an XbaI digested ML123 plasmid. This one has a replication origin derived from pVS1, which allows replication in Xanthomonas, Pseudomonas and Ralstonia. To determine whether the biobrick had ligated in the opposite direction to the strong promoter npt (Figure 2), transformed colonies were confirmed via enzymatic digestions. This way the biobrick would not be induced by the npt promoter present in the plasmid, and therefore, the fluorescent response due to the presence of 3OH-PAME would depend solely on PxpsR.
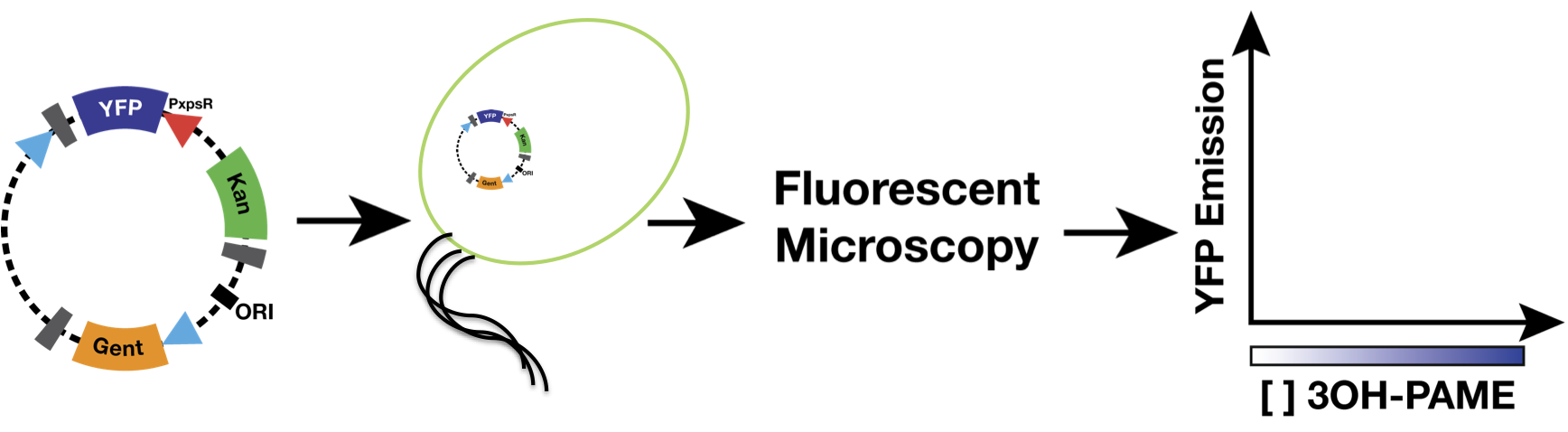
We used as host a mute mutant of Ralstonia Solanacearum, which has a mutation in the gene phcB (3 OH-PAME synthetase). Although it has the ability to sense 3 OH-PAME, it cannot produce it (Figure 3), which allows to create an environment with a known concentration of 3 OH-PAME at all times, indepent of the number of bacterias present. We are currently transforming the mute strain with the PxpsR-eYFP reporter in pML123, once we obtain the transformants we will determine the fluorescent response at different concentrations of synthetic 3-OH-PAME (Figure 4). Based on the fluorescent response, we will be able to determine the inducibility of PxpsR.
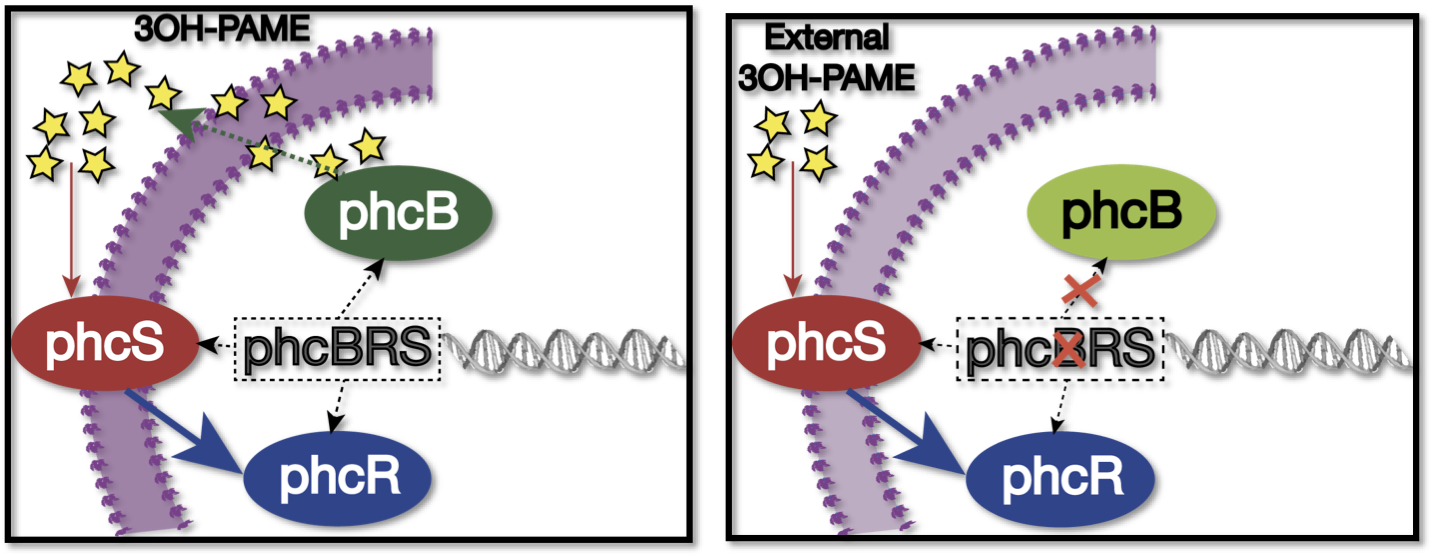
 "
"