Team:Marburg SYNMIKRO/Notebook
From 2012.igem.org
(Difference between revisions)
Enantiomere (Talk | contribs) |
Enantiomere (Talk | contribs) |
||
| Line 943: | Line 943: | ||
: parameters: 98°C 5min, >> 98°C 1min, 72°C 1min << x30, 72°C 5min, 4°C ∞ | : parameters: 98°C 5min, >> 98°C 1min, 72°C 1min << x30, 72°C 5min, 4°C ∞ | ||
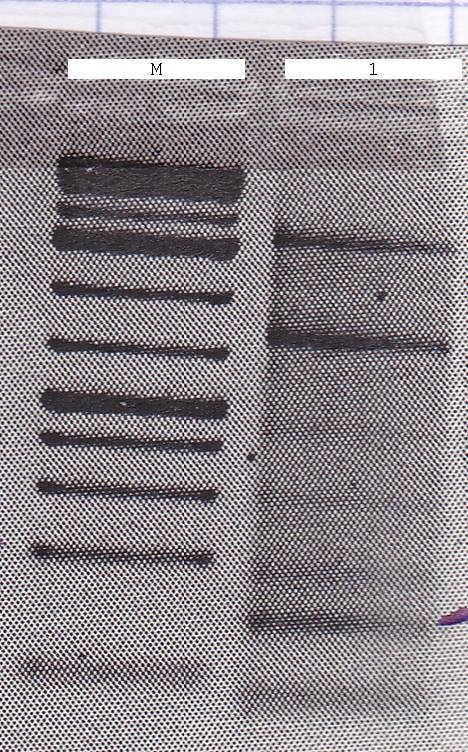
| - | ; Agarose gel of backbone PCR | + | ; Agarose gel of backbone PCR |
: no result | : no result | ||
; elution of the backbones of Friday the 24th | ; elution of the backbones of Friday the 24th | ||
| - | ; Agarose gel | + | |
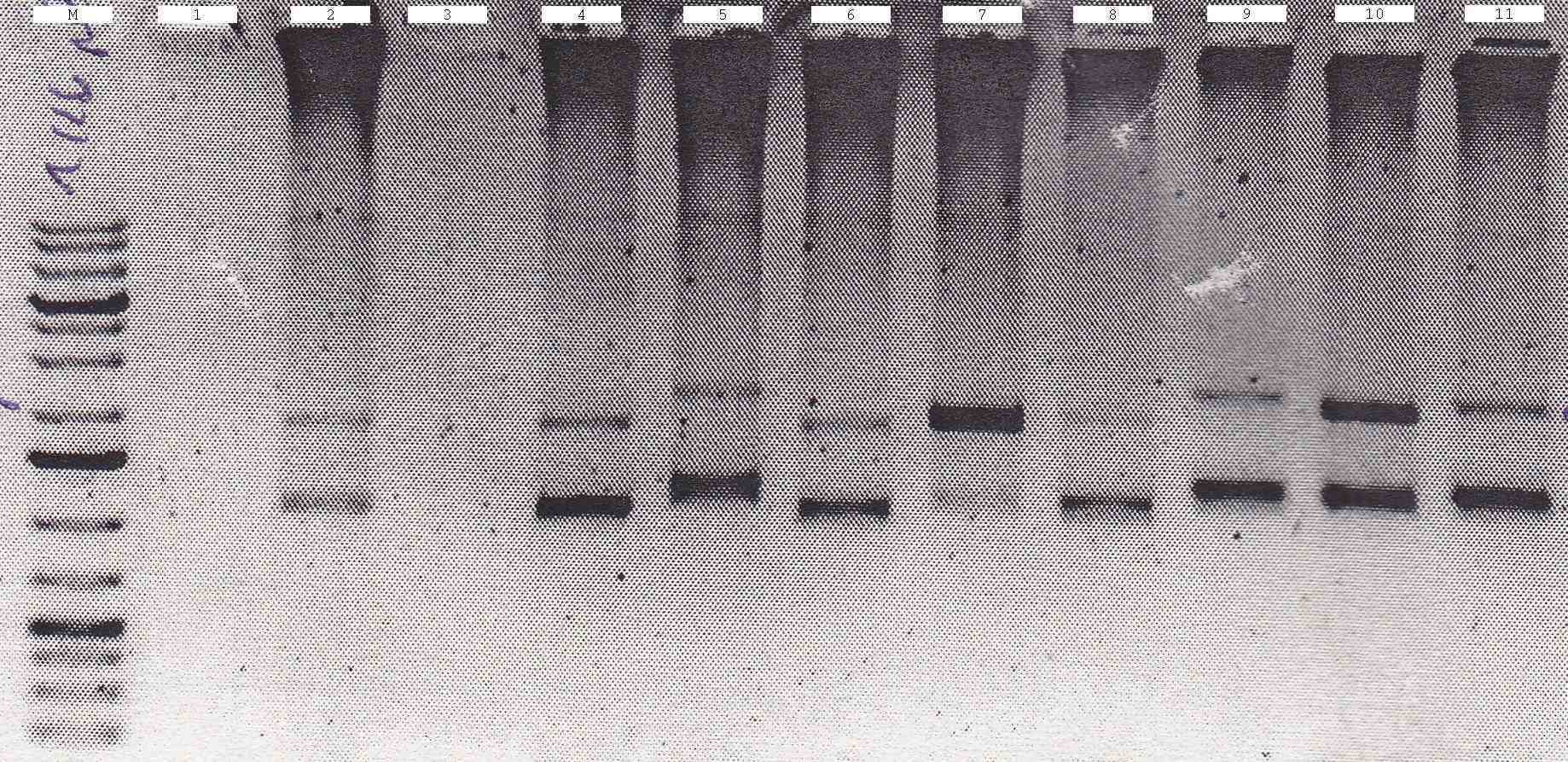
| - | : | + | ; 1% Agarose gel |
| + | : [[File:Syn_MR_Gel33.jpg|thumb|800px|center|M:1kb gene ruler plus, 1-2:gixR, 3-9: LacIQ-RBS-Gin, 10: CFP, (colony PCR 27.08.2012)]] | ||
| + | |||
| + | |||
| + | ; 1% Agarose gel | ||
| + | : [[File:Syn_MR_Gel34.jpg|thumb|800px|center|M:1kb gene ruler plus, 1-4:CFP, 5-10: mRFP, (colony PCR 27.08.2012)]] | ||
; Inoculation | ; Inoculation | ||
| Line 965: | Line 970: | ||
: Enhancer, Terminator, Gix, P-RBS | : Enhancer, Terminator, Gix, P-RBS | ||
: parameters: 98°C 5', 98°C 1', 66°C 30", 72°C 30", 72°C 5', 4°C ~ | : parameters: 98°C 5', 98°C 1', 66°C 30", 72°C 30", 72°C 5', 4°C ~ | ||
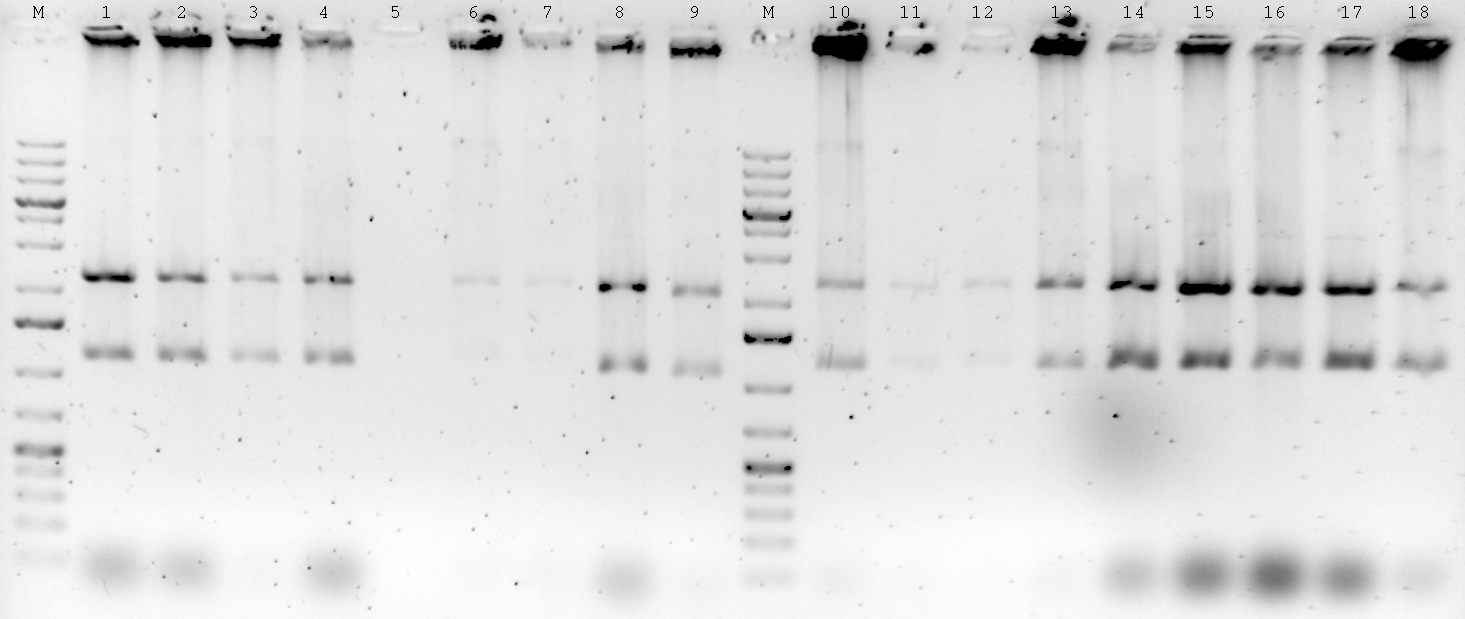
| - | : | + | |
| + | ; 1% Agarose gele | ||
| + | : [[File:Syn_MR_Gel35.jpg|thumb|800px|center|M:1kb gene ruler plus, 1:Terminator, 2-3: Enhancer, 4-6: P-RBS, 7-8: GixR, 9-10: Enhancer, (PCR 28.08.2012)]] | ||
; Transformation | ; Transformation | ||
| Line 986: | Line 993: | ||
: diluted templates: Terminator, P-RBS, Gix 1 --> 1:10 | : diluted templates: Terminator, P-RBS, Gix 1 --> 1:10 | ||
: Enhancer, Gix 2 --> 1:20 | : Enhancer, Gix 2 --> 1:20 | ||
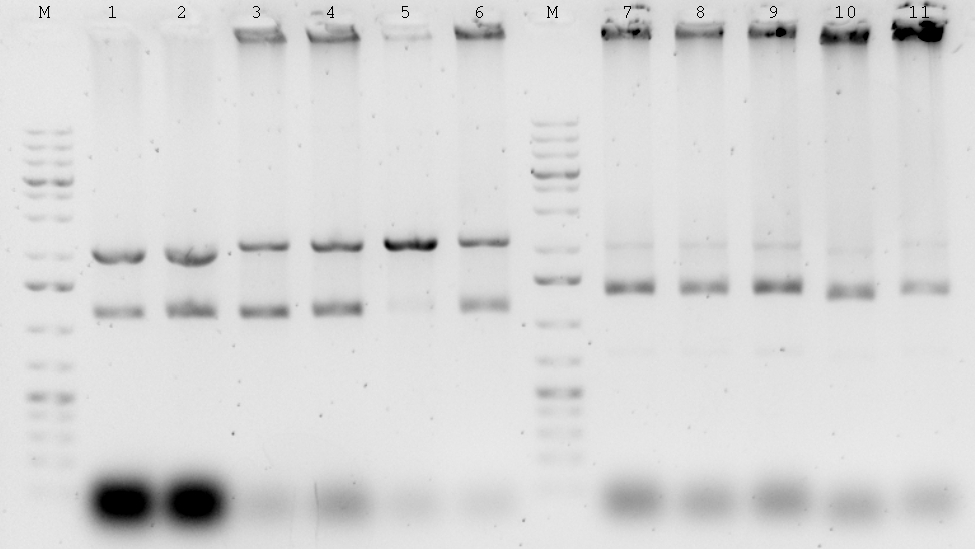
| - | : | + | |
| + | ; 1% Agarose gele | ||
| + | : [[File:Syn_MR_Gel36.jpg|thumb|800px|center|M:1kb gene ruler plus, 1:Terminator, 2-3: Enhancer, 4-6: P-RBS, 7-8: GixR, 9: Enhancer control, 10: P-RBS control, 11: GixR control, (PCR 29.08.2012)]] | ||
; 3AA | ; 3AA | ||
| Line 1,015: | Line 1,024: | ||
; Sequencing | ; Sequencing | ||
| - | : | + | : CFP_fwd |
| - | + | : CFP_rev | |
| + | : mRFP_fwd | ||
| + | : mRFP_rev | ||
| + | : P-RBS_fwd | ||
| + | : GixR_fwd | ||
| + | : GixR_fwd | ||
| + | : Enhancer_fwd | ||
== 30.08.2012 == | == 30.08.2012 == | ||
| Line 1,035: | Line 1,050: | ||
: Parameters No.2: 95°C 5', >>95°C 30", 72°C 1.5'<< x30, 72°C 5', 4°C | : Parameters No.2: 95°C 5', >>95°C 30", 72°C 1.5'<< x30, 72°C 5', 4°C | ||
| - | ; Agarose | + | ; 1% Agarose gele |
| - | : | + | : [[File:Syn_MR_Gel37.jpg|thumb|800px|center|M:1kb gene ruler plus, 1,3,5:SacB, 4,5: none, (PCR 31.08.2012)]] |
| - | : | + | |
; Ligation | ; Ligation | ||
| Line 1,093: | Line 1,107: | ||
| - | ; PCR | + | ; colony-PCR |
: of colonies (CFP-T, mRFP-T) | : of colonies (CFP-T, mRFP-T) | ||
: parameters: 98°C 5', >>98°C 1', 72°C 1'<<x30, 72°C 5', 4°C | : parameters: 98°C 5', >>98°C 1', 72°C 1'<<x30, 72°C 5', 4°C | ||
| Line 1,102: | Line 1,116: | ||
: pC3-CFP (14) | : pC3-CFP (14) | ||
: pC3.mRFP (24) | : pC3.mRFP (24) | ||
| - | : | + | |
| + | ; 1% Agarose gele | ||
| + | : [[File:Syn_MR_Gel38.jpg|thumb|800px|center|M:1kb gene ruler plus, 1-6:CFP-Terminator, 7:CFP control, 8-13:mRFP-Terminator 14: mRFP control, (colony-PCR 03.09.2012)]] | ||
Revision as of 14:46, 26 September 2012

Contents |
Week 1
[all]
09.07. - 15.07.2012
09.07.2012
- Inoculation
- E. coli strain C600 Mucts62 in dYT-medium
10.07.2012
- Isolation of chromosomal DNA
- E. coli strain C600 Mucts62 (lysogenic for temperature sensitive phage Mu cts62)
- Inoculation
- E. coli strain DH5α and TOP10 in dYT-medium
11.07.2012
- Preparation of chemically competent E. coli cells
- TOP10
12.07.2012
- Transformation
- GFP, GFP_fusion, CFP, mRFP, RBS, P108, P100, Plac with E.coli TOP10
| Name | Biobrick | Plate | Well | Backbone | Resistance |
|---|---|---|---|---|---|
| GFP | [http://partsregistry.org/Part:BBa_E0040 BBa_E0040] | 1 | 14K | pSB1A2 | Ampicillin |
| GFP_fusion | [http://partsregistry.org/Part:BBa_K125500 BBa_K125500] | 3 | 2P | pSB1A2 | Ampicillin |
| CFP | [http://partsregistry.org/Part:BBa_E0020 BBa_E0020] | 1 | 6A | pSB1A2 | Ampicillin |
| mRFP | [http://partsregistry.org/Part:BBa_E1010 BBa_E1010] | 1 | 18F | pSB2K3 | Kanamycin |
| RBS | [http://partsregistry.org/Part:BBa_J61100 BBa_J61100] | 1 | 5J | pSB1A2 | Ampicillin |
| P108 | [http://partsregistry.org/Part:BBa_J23108 BBa_J23108] | 2 | 2E | BBa_J61002 | Ampicillin |
| P100 | [http://partsregistry.org/Part:BBa_J23100 BBa_J23100] | 1 | 18C | BBa_J61002 | Ampicillin |
| PLac | [http://partsregistry.org/Part:BBa_I14032 BBa_I14032] | 2 | 11P | pSB2K3 | Kanamycin |
Week 2
16.07. - 22.07.2012
16.07.2012
- PCR
- AmiC (primer: MS1 + MS2)
- HU (primer: MS3 + MS4)
- GroES (primer: MS5 + MS6)
- GixR (primer: MS7 + MS8)
- Enhancer (primer: MS9 + MS10)
- ⇒ template-DNA: chromosomal DNA, denaturation: 30 sec at 95°C; annealing: 30 sec at 60°C; elongation: 30 sec at 72°C; 35 cycles
- Inoculation
- GFP
- GFP_fusion
- CFP
- mRFP
- RBS
- P108
- LacIQ
- P100
17.07.2012
- Plasmid preparation
- GFP
- GFP_fusion
- CFP
- mRFP
- RBS
- P108
LacIq- P100
18.07.2012
- Agarose gel
- DNA Gel extraction
- Enhancer
- GroES
- HU
- GixR Hybridization
- 5 µl primer MS7 (100 µM) + 5 µl primer MS8 (100 µM)
- 5 min 99°C
- over night, room temperature
- -80°C
19.07.2012
- Fill-in reaction (primer extension)
- Enhancer
- Restriction
- Enhancer (EcoRI/PstI)
- PCR
- AmiC (primer: MS1 + MS2)
- ⇒ template-DNA: chromosomal DNA, annealing: 30 sec; 85 °C, elongation: 36 sec; 72 °C, 35 cycles, 1) 2 µl DMSO; 1 µl chrom. DNA | 2) 2 µl DMSO; 5 µl chrom. DNA | 3) without DMSO; 1 µl chrom. DNA | 4) without DMSO; 5 µl chrom. DNA | 5) 2 µl DMSO; without chrom. DNA
- Test restriction digest
- 7) CFP (EcoRI/PstI)
- 8) GFP (EcoRI/PstI)
- 9) GFP-fusion (EcoRI/PstI)
- 10) mRFP (EcoRI/PstI)
- ⇒ all negative
- Agarose gel
- Control digest
- CFP (EcoRI/PstI)
- GFP (EcoRI/PstI)
- GFP-fusion (EcoRI/PstI)
- mRFP (EcoRI/PstI)
- ⇒ restriction over night
20.07.2012
- Agarose gel from the control digest
Week 3
23.07. - 29.07.2012
26.07.2012
- Restriction
- pSB1T3 (XbaI/SpeI and DpnI)
- pSB1A3 (XbaI/SpeI and DpnI)
- pSB1K3 (XbaI/SpeI and DpnI)
- pSB1C3 (XbaI/SpeI and DpnI)
- Ligation
- GixR + pSB1A3
- Enhancer + pSB1K3
- cyclisation of pSB1C3, pSB1K3, pSB1A3, pSB1T3 (couldn’t work)
Week 4
30.07. - 05.08.2012
30.07.2012
- PCR
- CFP (primer: MS16 + MS17) ⇒ template-DNA: BBa_E0010
- ⇒ positive
- mRFP (primer: MS14 + MS15) ⇒ template-DNA: BBa_E1010
- ⇒ positive
- Gin (primer: MS11 + MS13) ⇒ template-DNA: chromosomal DNA (that was wrong)
- RBS-Gin (primer: MS13 + MS12) ⇒ template-DNA: chromosomal DNA (that was wrong)
- ⇒ annealing: 30 sec; 60 °C, elongation: 40 sec; 72 °C, 35 cycles
- Transformation
- all vector-backbone-ligations
- Enhancer (K3)
- GixR (A3)
31.07.2012
- Result
- transformation
- pSB1A3: 3 colonies
- pSB1K3: 60 colonies
- pSB1C3: 9 colonies
- pSB1T3: 0 colonies
- Enhancer (K3): 0 colonies
- GixR (A3): 0 colonies
- Agarose gel
- Agarose gel
- Agarose gel
- Resuspension of a biobrick
- BBa_B0010 (terminator)
- DNA Gel extraction
- CFP
- mRFP
- PCR
- AmiC (primer: MS1 + MS2)
- RBS-Gin (primer: MS12 + MS13)
- Gin (primer: MS11 + MS13)
- ⇒ template-DNA: chromosomal DNA, annealing: 30 sec; 55 °C, elongation: 40 sec; 72 °C, 35 cycles
- Restriction
- pSB1A3 (EcoRI/PstI)
- CFP (EcoRI/PstI)
- mRFP (EcoRI/PstI)
- Transformation
- pSB1A3
- pSB1C3
- pSB1T3
- Terminator
- GixR
- Enhancer
- Inoculation
- pSB1A3
- pSB1C3
- pSB1K3
- Ligation
- pSB1A3 + CFP
- pSB1A3 + mRFP
- GixR Hybridization
- 5 µl primer MS7 (100 µM) + 5 µl primer MS8 (100 µM)
- 5 min 99°C
- over night, room temperature
- -80°C
- DNA Gel extraction
- AmiC
- Ligation
- AmiC + pSV2 (suicide vector)
01.08.2012
- Result
- transformation
- Only BBa_B0010 colonies present
- Plasmid preparation
- pSB1A3 ⇒ only clone 1, 3 Positive
- pSB1C3 ⇒ all negative
- pSB1K3 ⇒ all negative
- Ligation
- GixR hyb. + pJET2_1
- ⇒ over blunt ends
- Restriction
- GixR hyb. (EcoRI/PstI)
- pSB1C3 (EcoRI/PstI)
- Agarose gel
- Agarose gel
- DNA Gel extraction
- RBS-Gin
- Gin
- AmiC
- Test restriction
- pSB1A3 (EcoRI)
- Restriction
- RBS-Gin (EcoRI/PstI)
- Gin (EcoRI/PstI)
- AmiC (EcoRI/PstI)
- pSB1C3 (EcoRI/PstI)
- pSB1K3 (EcoRI/PstI)
- pSB1T3 (EcoRI/PstI)
- Ligation
- cyclisation of pSB1T3
- Fill-in reaction (primer extension)
- Enhancer
- Ligation
- Enhancer and GixR in pSV2
- Enhancer in pSB1K3
- GixR in pSB1C3
- Agarose gel
- pSB1A3 c2
- pSB1C3 c4
- pSB1K3 c3
- Transformation
- pSB1A3 religation
- CFP in A3
- mRFP in A3
- AmiC in pJET
- GixR in pJET
- Enhancer in pJET
- GixR in C3
- Enhancer in K3
- Ligation
- AmiC + pSB1T3
- RBS-Gin + pSB1K3
- Gin + pSB1K3
02.08.2012
- Resuspension of a biobrick
- pSB3C5
- ⇒ for ccdB
- Plasmid preparation
- BBa_B0010 (terminator)
- Test restriction
- Terminator c1,2,3,4 (EcoRI/PstI)
- 2% Agarose gel
- Transformation
- pSB1T3 recycled
- AmiC in T3
- Gin-RBS in K3
- Gin in K3
- pSB3C5 linear (false)
03.08.2012
- Plasmid preparation
- Enhancer in pJET
Week 5
06.08. - 12.08.2012
06.08.2012
- Resuspension and transformation of a biobrick
- BBa_J04450 in pSB1A3
- BBa_J04450 in pSB1C3
- BBa_J04450 in pSB1K3
- BBa_J04450 in pSB1T3
- Restriction
- GFP-fusion (EcoRI/SpeI)
- Terminator (XbaI/PstI)
- Promoter BBa_J23100 (EcoRI/SpeI)
- Promoter BBa_J23108 (EcoRI/SpeI)
- RBS (XbaI/PstI)
- Ligation
- RBS-Gin in T3
- Gin in T3
- GFP-fusion (EcoRI/SpeI) + Terminator (XbaI/PstI) in pSB1K3
- Promoter BBa_J23108 (EcoRI/SpeI) + RBS (XbaI/PstI) in pSB1K3
- Promoter BBa_J23100 (EcoRI/SpeI) + RBS (XbaI/PstI) in pSB1K3
- GixR Hybridization
- 1(3) µl primer MS7 (100 µM) + 1(3) µl primer MS8 (100 µM) + T4-Ligasebuffer
- 5 min 95°C
- over night, room temperature
- -80°C
- Transformation
- GixR in C3
- Gin in T3
- RBS-Gin in T3
- mRFP in A3
- CFP in A3
07.08.2012
- Transformation
- GFP-fusion
- CFP
- J23108-RBS
- J23100-RBS
- Plasmid preparation
- GixR 1,2,3
- Restriction
- GixR hyb. 1,2,3 (EcoRI/PstI)
- pSB1C3 (EcoRI/PstI)
- Ligation
- GixR hyb. 1,2,3 in pJET
- GixR hyb. 1,2,3 in C3
- Transformation
- GixR hyb. 1,2,3 in pJET
- GixR hyb. 1,2,3 in C3
- Test restriction
- pSB3C5 (XbaI)
- pJET (XbaI)
- 1% Agarose gel
- ⇒ positive: pSB3C5 3,4,7,8,; Enhancer-pJET 9-12
08.08.2012
- Result transformation
- GFP-fusion (20 clones)
- J23108-RBS (20 clones)
- J23100-RBS (20 clones)
- GixR hyb. 1,2,3 in pJET (10 clones)
- GixR hyb. 1,2,3 in C3 (10 clones)
- CFP (5 clones)
- Restriktion
- Enhancer in pJET (EcoRI/PstI)
- pSB1C3 (EcoRI/PstI)
- 2% Agarose gel
- DNA Gel extraction
- Enhancer
- pSB1C3
- Ligation
- Enhancer 1,2 in C3
- Transformation
- Enhancer 1,2 in C3
- C3
- mRFP
09.08.2012
- Result transformation
- GFP-fusion ⇒ positive
- J23108-RBS ⇒ positive
- J23100-RBS ⇒ positive
- CFP ⇒ positive
- GixR in C3 c1 ⇒ negative
- GixR in C3 c2 ⇒ negative
- GixR in C3 c3 ⇒ negative
- Plasmid preparation
- GFP-fusion
- J23108-RBS
- J23100-RBS
- CFP
- Test restriction
- GFP-fusion (EcoRI/PstI)
- J23108-RBS (EcoRI)
- J23100-RBS (EcoRI)
- CFP (EcoRI/PstI)
- 1% Agarose gel
- 1% Agarose gel
- 1% Agarose gel
- 1% Agarose gel
- Inoculation
- GixR in C3
- Gin in T3
- Gin-RBS in T3
- pSB1C3
- Enhancer in C3
- mRFP in A3
Week 6
13.08. - 19.08.2012
13.08.12
- PCR
- GroES (primer: MS5 + MS6 (b))
- HU (primer: MS3 + MS4 (b))
- ⇒ with and without DMSO, template-DNA: chromosomal DNA, annealing: 30 sec; 60 °C, elongation: 40 sec; 72 °C, 35 cycles
- 1% Agarose gel
- Transformation
- LacIQ (BBa_I14032)
14.08.12
- Restriction
- pSB1K3 (EcoRI/PstI)
- Enhancer 3,4 (EcoRI/ PstI)
- GFP-fusion (EcoRI/SpeI)
- Terminator (XbaI/PstI)
- Test restriction
- RBS-Gin in T3 (EcoRI/ PstI)
- Ligation
- GFP-fusion (EcoRI/SpeI) + Terminator (XbaI/PstI) in pSB1K3 (EcoRI/PstI)
- Promotor J23108 (EcoRI/SpeI) + RBS (XbaI/PstI) in pSB1K3 (EcoRI/PstI)
- Enhancer 3,4 (EcoRI/ PstI) + pSB1K3 (EcoRI/PstI)
- Transformation
- GFP-fusion-Terminator in pSB1K3
- Promotor-RBS in pSB1K3
- Enhancer 3 in pSB1K3
- Enhancer 4 in pSB1K3
- GixR in pJET
15.08.2012
- PCR
- GroES (primer: MS5 + MS6 (b))
- HU (primer: MS3 + MS4 (b))
- ⇒ with and without DMSO, template-DNA: chromosomal DNA, annealing: 30 sec; 55 °C, elongation: 30 sec; 72 °C, 35 cycles
- 1% Agarose gel
- DNA Gel extraction
- HU 1,2
- Resuspension of a biobrick
- BBa_J04450 in pSB1A3
- BBa_J04450 in pSB1T3
- BBa_J04450 in pSB1C3
- BBa_J04450 in pSB1K3
- Transformation
- BBa_J04450 in pSB1A3
- BBa_J04450 in pSB1T3
- BBa_J04450 in pSB1C3
- BBa_J04450 in pSB1K3
- Test restriction
- pSB1T3 (EcoRI/PstI and DpnI)
- pSB1K3 (EcoRI/PstI and DpnI)
- pSB1C3 (EcoRI/PstI and DpnI)
- pSB1A3 (EcoRI/PstI and DpnI)
16.08.2012
- PCR
- GroES (primer: MS5 + MS6 (b))
- ⇒ with and without DMSO, template-DNA: chromosomal DNA, annealing: 30 sec; 55 °C, elongation: 30 sec; 72 °C , 35 cycles
- 1% Agarose gel
- Plasmid preparation
- P-RBS
- Test restriction
- P-RBS (EcoRI/PstI)
- 1% Agarose gel
- Restriction
- Enhancer 3,4 (EcoRI/ PstI)
- HU (EcoRI/ PstI)
- AmiC (EcoRI/ PstI)
- Promoter, GFP (EcoRI/SpeI)
- RBS; Terminator (XbaI/PstI)
- Ligation
- HU, AmiC in pSB1A3
- Enhancer 3,4 in pSB1K3
- P-RBS in pSB1K3
- GFP fusion-Terminator in pSB1K3
- DNA Gel extraction
- GroES 1, P-RBS
- Transformation
- AmiC
- HU
- Enhancer 3,4, 3 alt,4 alt
- Inoculation
- BBa_J04450 in pSB1A3
- BBa_J04450 in pSB1T3
- BBa_J04450 in pSB1C3
- BBa_J04450 in pSB1K3
- AmiC
- P-RBS
17.08.2012
- Restriction
- GroES (EcoRI/SpeI)
- Ligation
- GroES in pSB1C3
- Transformation
- GroES in pSB1C3
- Test restriction
- P-RBS (EcoRI/PstI)
- BBa_J04450 in pSB1A3 (EcoRI/PstI)
- BBa_J04450 in pSB1T3 (EcoRI/PstI)
- BBa_J04450 in pSB1C3 (EcoRI/PstI)
- BBa_J04450 in pSB1K3 (EcoRI/PstI)
- 1% Agarose gel
- Agarose gel
- pipet scheme
- marker|P-RBS 1,2,3,4| P-RBS 1,2,3,4| marker| BBa_J04450 in pSB1K3 1,2,3,4
(bild 22)
- 1% Agarose gel
Week 7
20.08. - 26.08.2012
20.08.2012
- PCR
- Backbone (primer MS24 + MS25)
- ⇒ template-DNA: pSB1A3, pSB1T3, pSB1C3 and pSB1K3, annealing: 30 sec; 55 °C, elongation: 30 sec; 72 °C , 35 cycles
- 1% Agarose gel
- Transformation
- BBa_JH023
- Inoculation
- GroES
- P-RBS
- GFP-T
- Enhancer 3,4
- AmiC
- P-RBS alt
- GFP-T alt
21.08.2012
- Results
- colonies of BBa_JH023
- inoculations except AmiC and P-RBS alt successful
- PCR
- pSB1A3-BBa_J04450 (template DNA)
- pSB1T3-BBa_J04450 (template DNA)
- pSB1C3-BBa_J04450 (template DNA)
- pSB1K3-BBa_J04450 (template DNA)
- used primer --> MS24Prefix_rev + MS25Suffix_fwd
- initial denaturation 98°C - 5min
- denaturation: 98°C - 1min; annealing: 55°C - 30s; elongation: 72°C - 2.5min -->repeat 35 times
- 72°C - 5min --> 4°C - ∞
- miniprep
- GroES, Enh 3/4 (+old), P-RBS (+old), GFP-T (+old)
- Test restriction
- of miniprep
- over night, 37°C
- 1% Agarose gel
- Inoculation
- E.coli BBa_I14032
- over night, 37°C
22.08.2012
- PCR
- CFP
- mRFP
- initial denaturation 98°C - 5min
- denaturation: 98°C - 10s; annealing: 60°C - 30s; elongation: 72°C - 40s -->repeat 35 times
- 72°C - 5min --> 4°C - ∞
- 1% Agarose gel
- miniprep
- of LacIQ
- Test restriction
- of LacIQ with EcoRI and PstI
- 1% Agarose gel
- 1% Agarose gel
- 1% Agarose gel
- 1% Agarose gel
- test restriction of Enhancer (->21.08.12), gel 2%
- watch picture
- test restriction of Enhancer and P-RBS (->21.08.12), gel 2%
- watch picture
23.08.2012
- PCR
- pSB1K3-BBa_J04450 (template DNA)
- pSB1T3-BBa_J04450 (template DNA)
- used primer --> MS24Prefix_rev + MS25Suffix_fwd
- initial denaturation 98°C - 5min
- denaturation: 98°C - 1min; annealing: 55°C - 30s; elongation: 72°C - 2.5min -->repeat 35 times
- 72°C - 5min --> 4°C - ∞
- 1% Agarose gel
- Restriction
- GixR (EcoRI/PstI)
- Ligation
- GixR in pSB1C3
- Transformation
- GixR in pSB1C3
24.08.2012
- PCR
- pSB1A3-BBa_J04450 (template DNA)
- pSB1T3-BBa_J04450 (template DNA)
- pSB1C3-BBa_J04450 (template DNA)
- pSB1K3-BBa_J04450 (template DNA)
- initial denaturation 98°C - 5min
- denaturation: 98°C - 10s; annealing: 60°C - 30s; elongation: 72°C - 40s -->repeat 30 times
- 72°C - 10min --> 4°C - ∞
- primer: MS22, MS23
- 1% Agarose gel
- Restriction
- RBS (XbaI/PstI)
- CFP (EcoRI/PstI)
- mRFP (EcoRI/PstI)
- Ligation
- mRFP und CFP in pSB1C3
- Transformation
- mRFP und CFP in pSB1C3
Week 8
27.08. - 02.09.2012
27.08.2012
- Results
- CFP: 1 colony
- CFP (neu): Colonies
- mRFP: colonies
- mRFP (neu): no colonies
- PCR
- template: backbones (pSB1K3/T3/C3/A3
- parameters: 98°C 5min, >> 98°C 1min, 70°C 30s, 72°C 2.5min << x30, 72°C 5min, 4°C ∞
- colony-PCR
- parameters: 98°C 5min, >> 98°C 1min, 72°C 1min << x30, 72°C 5min, 4°C ∞
- Agarose gel of backbone PCR
- no result
- elution of the backbones of Friday the 24th
- 1% Agarose gel
- 1% Agarose gel
- Inoculation
- pSB1C3_...
- mRFP clone 20,24
- CFP clone 14,15,17,18
- gix clone 1,2
- 5ml LB, over night, 37°C
28.08.2012
- production of competent TOP10
- PCR
- Enhancer, Terminator, Gix, P-RBS
- parameters: 98°C 5', 98°C 1', 66°C 30", 72°C 30", 72°C 5', 4°C ~
- 1% Agarose gele
- Transformation
- pBad, araC, pSacB
- over night, 37°C
- miniprep
- GixR (1,2)
- CFP (14,15,17,18)
- mRFP (20,24)
- alle in pSB1C3
29.08.2012
- Results
- Transformation: no colonies
- PCR
- Terminator, Gix, Enhancer, P-RBS
- diluted templates: Terminator, P-RBS, Gix 1 --> 1:10
- Enhancer, Gix 2 --> 1:20
- 1% Agarose gele
- 3AA
- CFP, mRFP with Terminator
- 2µl DNA
- 1µl SpeI
- 2µl Tango
- 15µl dH2O
- --> 1h at 37°C
- +1µl EcoRI
- +2,5µl Tange
- --> 1h at 37°C
- --> inactivation for 20min at 80°C
- Ligation
- 3AA products in pSB1T3
- 18.5µl H2O
- 2.5 µl Buffer
- 1µl Insert CFP or mRFP (EcoRI + SpeI)
- 1µl Insert Terminator (BBa_B0010) (XbaI + PstI)
- 1µl pSB1T3 (EcoRI + PstI)
- 1µl Ligase
- --> 1h at RT
- Transformation
- pBad --> TOP10 + pSB2K3
- CFP-T, mRFP-T --> TOP10 + pSB1T3
- Sequencing
- CFP_fwd
- CFP_rev
- mRFP_fwd
- mRFP_rev
- P-RBS_fwd
- GixR_fwd
- GixR_fwd
- Enhancer_fwd
30.08.2012
- Results
- Transformation: no colonies --> testing Trafo with BBa_J04450 A/K/T/C
- 2 colonies at pBad-Trafo from 28.08.2012 --> liquid culture for miniprep
- 1 colony at pSacB-Trafo from 28.08.2012 --> tested on succrose-sensitivity
31.08.2012
- PCR
- SacB
- Parameters No.1: 95°C 5', >>95°C 30", 60°C 30", 72°C 1'<< x30, 72°C 5', 4°C
- Parameters No.2: 95°C 5', >>95°C 30", 72°C 1.5'<< x30, 72°C 5', 4°C
- 1% Agarose gele
- Ligation
- 3AA (CFP-T, mRFP-T)
- watch 29.08.
- used vector: pSB1K3/T3
- Transformation
- of ligation in TOP10
- miniprep
- pBad/araC K.1+2
- restriction
- GixR rehybridization
- cut with EcoRI and PstI
- ligation
- 1µl GixRhyb E+P
- 1µl pSB1C3 E+P
- 1µl Th-Pol
- 2µl Buffer
- 15µl dH2O
- Transformation
- ligation + TOP10 --> LB(cam)
- Results of test-trafo
- Amp, Km, Cm OK --> Km with fewer colonies
- Tet --> hardly colonies
- --> comp.TOP10 obviously all right, exclusion of Tet in future work
Week 9
03.09. - 09.09.2012
03.09.2012
- Results
- Transformation
- GixR in C3 --> no colonies
- CFP-T/mRFP-T in C3(31.8.) --> no colonies
- CFP-T/mRFP-T in T3(31.8.) --> many but small colonies
- CFP-T/mRFP-T in T3(29.8.) --> less colonies
- colony-PCR
- of colonies (CFP-T, mRFP-T)
- parameters: 98°C 5', >>98°C 1', 72°C 1'<<x30, 72°C 5', 4°C
- Agarose gel
- 6x colony pT3-CFP-T from 29./31.8.
- 6x colony pT3-mRFP-T from 29./31.8.
- pC3-CFP (14)
- pC3.mRFP (24)
- 1% Agarose gele
Week 10
10.09. - 16.09.2012
11.09.2012
- microscopy-screening
- GroES-GFP ⇒; no fluorescence
- GroES-CFP ⇒; no fluorescence
- AmiC-mRFP ⇒; no fluorescence
- AmiC-GFP ⇒; no fluorescence
- colony-PCR
- GixR
- RBS-Gin
- Terminator
- 3A-Assembly (digestion, ligation, transformation)
- GroES (E/S) + Terminator (X/P) + pSB1K3 (P/E)
- AmiC (E/S) + Terminator (X/P) + pSB1K3 (P/E)
- plasmid-prep and control-digestion (E/P)
- GroES-GFP
- GroES-CFP
- AmiC-mRFP
- AmiC-GFP
- GroES-Terminator
- Enhancer
- GFP-Terminator
- Terminator
Week 11
17.09. - 23.09.2012
17.09.2012
- Restriction
- AmiC
- GroES
- CFP
- SacB
- CFP-C3
- SacB (2nd try)
- for results see pictures
- Resuspend
- BBa_B001J
- Restreak
- RBS-Gin (HF)
- RBS-Gin (GC)
- RBS-Gin (SM)
- DNA isolation
- CFP-C3 (Miniprep28.08.12)--> 47,3 ng/µl
- Miniprep
- GixR pSB1K3 --> 50,5 ng/µl, 144,7 ng/µl
- RBS(new)B0030 pSB1A2 --> 726,2 ng/µl, 242,6 ng/µl
- P108-RBS pSB1C3 --> 200,5 ng/µl, 177,8 ng/µl
- for results see picture
- Transformation
- pSB2K3-pBAD & pSB1-A2-TT ???????? kann ich nicht lesen
- Agarose gel
- CFP-C3 (restriction 2nd try)
- SacB-Cs (restriction 2nd try)
- for results see picture
18.09.2012
- Results
- Transformation
- 2x Terminator --> colonies
- pBAD --> no colonies
- Colony PCR
- RBS-Gin
- 2x Terminator
- for results see picture
- Sequencing
- SacB
- Gel extraction
- CFP-C3 E+X
- Ligation
- am-G: amiC E+S & GFP-A3 E+X
- am-C: amiC E+S & CFP-C3 E+X
- am-R: amiC E+S & mRFP-C3 E+X
- gro-G: groES E+S & GFP-A3 E+X
- gro-C: groES E+S & CFP-C3 E+X
- gro-R: groES E+S & mRFP-C3 E+X
- Transformation
- am-G
- am-C
- am-R
- gr-G
- gr-C
- gr-R
- pBAD
19.09.2012
- Results
- Transformation
- am-G --> colonies
- am-C --> colonies
- am-R --> colonies
- gr-G --> colonies
- gr-C --> colonies
- gr-R --> colonies
- pBAD --> no colonies
- Colony PCR
- RBS-Gin (from 17.09.12)
- 2x Terminator B0017 (from 17.09.12)
- --> Amplification failed
- Transformation
- pBAD
- Microscopy-screening
- AmiC-GFP --> fluorescence
- GroES-GFP --> fluorescence
- AmiC-CFP --> fluorescence
- GroES-CFP --> fluorescence
- AmiC-mRFP --> fluorescence
- GroES-mRFP --> fluorescence
- Inoculation
- RBS-Gin
- TT (B0017)
- AmiC-GFP
- GroES-GFP
- AmiC-CFP
- GroES-CFP
- AmiC-mRFP
- GroES-mRFP
20.09.2012
- Miniprep
- RBS-Gin
- TT (B0017)
- AmiC-GFP
- GroES-GFP
- AmiC-CFP
- GroES-CFP
- AmiC-mRFP
- GroES-mRFP
- for results see picture
- Restriction
- SacB
- P-RBS
- for results see picture
- Inoculation
- GroES-GFP
- GroES-CFP
- GroES-mRFP
21.09.2012
- Restriction
- GFP-fusion (E+S)
- TT BBa_0017 (E+X)
- for results see picture
- Ligation
- GFP bb Enhancer
- GFP TT
- GroES TT
- Transformation
- GFP bb Enhancer
- GFP TT
- GroES TT
- Promotor-RBS (17.09.12)
- Sequencing
- RBS-Gin
- Microscopy-screening
- AmiC-GFP --> Channel: DIC, Green, Red, Blue
- GroES-GFP --> Channel: DIC, Green, Red, Blue
- AmiC-CFP --> Channel: DIC, Green, Red, Blue
- GroES-CFP --> Channel: DIC, Green, Red, Blue
- AmiC-mRFP --> Channel: DIC, Red
- GroES-mRFP --> Channel: DIC, Green, Red, Blue
22.09.2012
- Inoculation
- GroES-TT pSB1A2
- GFP-TT pSB1A2
- Enhancer
- P108-RBS pSB1C3
 "
"