Team:Amsterdam/extra/diary
From 2012.igem.org
| Line 11: | Line 11: | ||
<table> | <table> | ||
<tr> | <tr> | ||
| - | <td colspan='5' | + | <td colspan='5' align='center'><b>Table of Contents</b></td> |
</tr> | </tr> | ||
<tr> | <tr> | ||
Revision as of 11:45, 26 September 2012




Diary
12/07/2012
Transformation of the plasmid containing the M.ScaI methyltransferase (pIDTSMART-AMP).
11/07/2012
Mini-prep BBa_J63009+BBa_J04450 and pSB1AT3+BBa_J45320. Elution in 25µl. Restriction digestion of BBa_J63009+BBa_J04450 with DraI and RsaI/XbaI. Restriction digestion of pSB1AT3+BBa_J45320 with ScaI and xhoI.
Something went wrong with the digestions.....
Transformation: 1)pSB3C5+ BBa_J04450 (2012 iGEM distribution kit plate 1 Well 3C) 2)pSB4C5+ BBa_J04450 (2012 iGEM distribution kit plate 1 Well 3E)
10/07/2012
Set overnight cultures for BBa_J63009+BBa_J04450 and pSB1AT3+BBa_J45320 for mini-prep.
09/07/2012
Symposium with all the dutch iGEM teams at Wageningen University.
No plates for pSB1C3 again.... Noooooooooo!
06/07/2012
Re-transform pSB1C3!
The overnight culture for BBa_J63009+BBa_J04450 and pSB1AT3+BBa_J45320 turned pink! What is going on?
At the end of the day -> BBa_J63009+BBa_J04450 and pSB1AT3+BBa_J45320 both contain RFP......the colonies should be reddish..... Oops....already threw them away. Awwwww start again then!
05/07/2012
Isolated colonies obtained for BBa_J63009+BBa_J04450 and pSB1AT3+BBa_J45320.
Set up overnight liquid LB culture for each plasmid for mini-prep.
04/07/2012
Plates show growth. Antibiotics are working! Swarming of bacteria. Unusable!
Streak new plates with a single colony to obtain isolated colonies. Incubate overnight at 37˚C.
Nothing grew on the pSB1C3 plate.
03/07/2012
Prepared ampicillin and chloramphenicol stock solutions (50 mg/ml).
Made LB/ampicillin and LB/chloramphenicol plates.
Transformation:
- pSB1AT3+BBa_J45320 (2012 iGEM distribution kit plate 2 Well 5J)
- BBa_J63009+BBa_J04450 (2012 iGEM distribution kit plate 1 Well 1A)
- pSB1C3 (2012 iGEM distribution kit linearised plasmid)
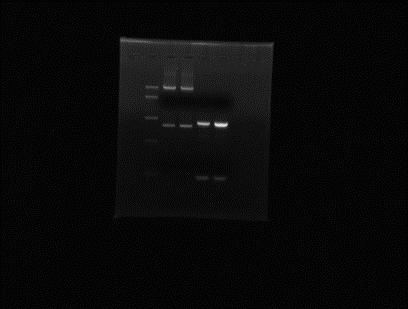
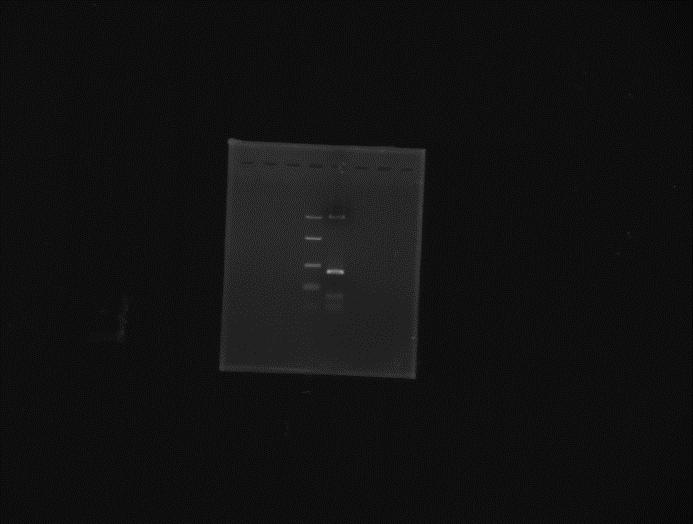
Incubate overnight at 37˚C.06/06/2012 Mini-prep of the colony obtained from Gibson on 05/06. Elution in 25µl miliQ water. Digestion of extracted plasmid with NotI and BglII (double digest).
Ladder Mini-prep colony digested with NotI and BglII
05/06/2012
One colony obtained. Checked for fluorescence with GFP. No fluorescence. Prepared overnight culture with colony for mini-prep.
01/06/2012
Diewertje removed the plates from the incubator and put them at 4˚C for the weekend. Thank you Diewertje!
31/05/2012
Prepared LB agar for LB plates + Kanamycin. Gibson assembly of peGFP and pET28a+ in equimolar amounts. Transformation of 5µl of Gibson reaction mix in DH5 alpha competent bacteria. Incubation overnight at 37˚C.
30/05/2012
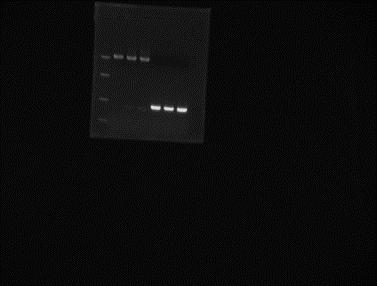
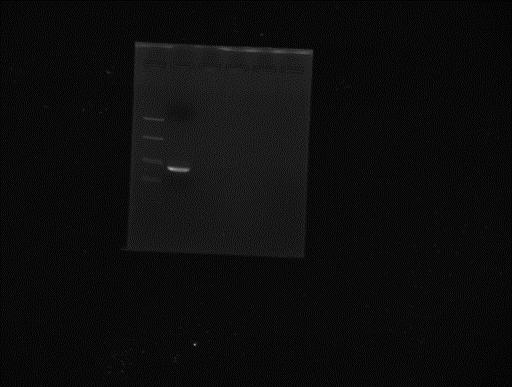
Prepared TAE and TBE gels with ethidium bromide. Pooled all the PCR products for peGFP and gel extract the correct band. Elution in 25µl miliQ water.
Ladder peGFP after gel extraction
Prepared the master mix for the Gibson assembly. Aliquoted 15µl in eppendorfs. Everything is ready! :)
29/05/2012
Gel electrophoresis of precipitated DNA pET28a+ from 25/05/2012
Ladder Gel extracted pET28a+ peGFP from 23/05
The pET28a+ is ready for the one step isothermal gibson assembly reaction. peGFP should also be gel extracted.
25/05/2012
Pooled all the PCR strips containing pET28a+ for DNA precipitation reaction.
24/05/2012
PCR pET28a+ -> annealing temperature 62˚C.
Ladder pET28a+
23/05/2012
Repeat what was done on 14/05 (PCR Temp 60˚C + gel extraction.
Two products obtained and concentration seems too low. Pfffffff.... Repeat again......And this is only a test, hope it will work better for the actual experiments
- (
16/05/2012
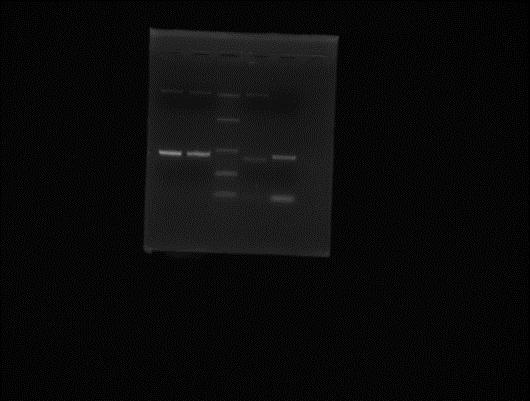
pET28a+ (gel extracted) peGFP (gel extracted) Ladder pET28a+ (PCR No.2 -> 14/05/2012) peGFP (PCR No.2 -> 14/05/2012)
Still non-specific products observed for the PCR. Possible inter-change during gel extraction; pET28a+ and peGFP got mixed up?
14/05/2012
PCR of pET28a+ and peGFP -> annealing temperature 56˚C. non-specific DNA products obtained for the vector. Increase annealing temperature? Gel extract?
Ladder Original pT28a+ Transformed pET28a+ (200 pg DNA for PCR) Transformed pET28a+ (1ng DNA for PCR) Original peGFP Transformed peGFP (200 pg DNA for PCR) Transformed peGFP (1 ng DNA for PCR)
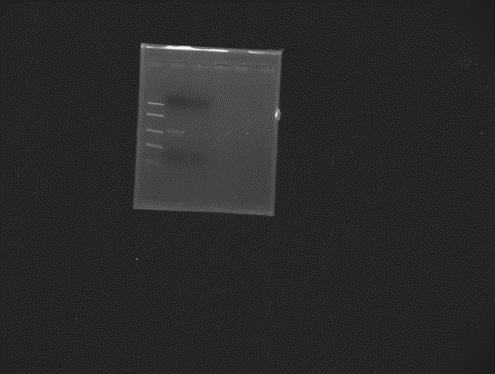
Gel extraction of pET28a+ from TAE gel + ethidium bromide. Elution in 25 µl miliQ water. PCR No. 2 -> pET28a+ and peGFP -> annealing temperature 60˚C.
11/05/2012
Gel electrophoresis of the PCR reaction performed on 10/05/2012. Non-specific products obtained. Repeat PCR? Change annealing temperature?
Ladder pET28a+ (1 ng for PCR) pET28a+ (10 ng for PCR) peGFP (1 ng for PCR) peGFP (10 ng for PCR)
Second PCR for peGFP and pET28a+. annealing temperature = 53˚C Same results obtained..... :(
 "
"