Team:SJTU-BioX-Shanghai/Project/project2.2
From 2012.igem.org
AleAlejandro (Talk | contribs) (→Design of membrane complex) |
(→Biosynthesis - Fatty Acid) |
||
| Line 27: | Line 27: | ||
===Background=== | ===Background=== | ||
| + | |||
Fatty acid derivatives are to emerge as prominent fuels in the marketplace. Their high energy density and low water solubility make them promising alternatives to fossil fuel as transportation fuels. Biosynthesis, among all the biofuels production methods, is the one most sustainable, environmental friendly and less controversial. | Fatty acid derivatives are to emerge as prominent fuels in the marketplace. Their high energy density and low water solubility make them promising alternatives to fossil fuel as transportation fuels. Biosynthesis, among all the biofuels production methods, is the one most sustainable, environmental friendly and less controversial. | ||
| Line 34: | Line 35: | ||
===Biosynthetic pathway=== | ===Biosynthetic pathway=== | ||
| + | |||
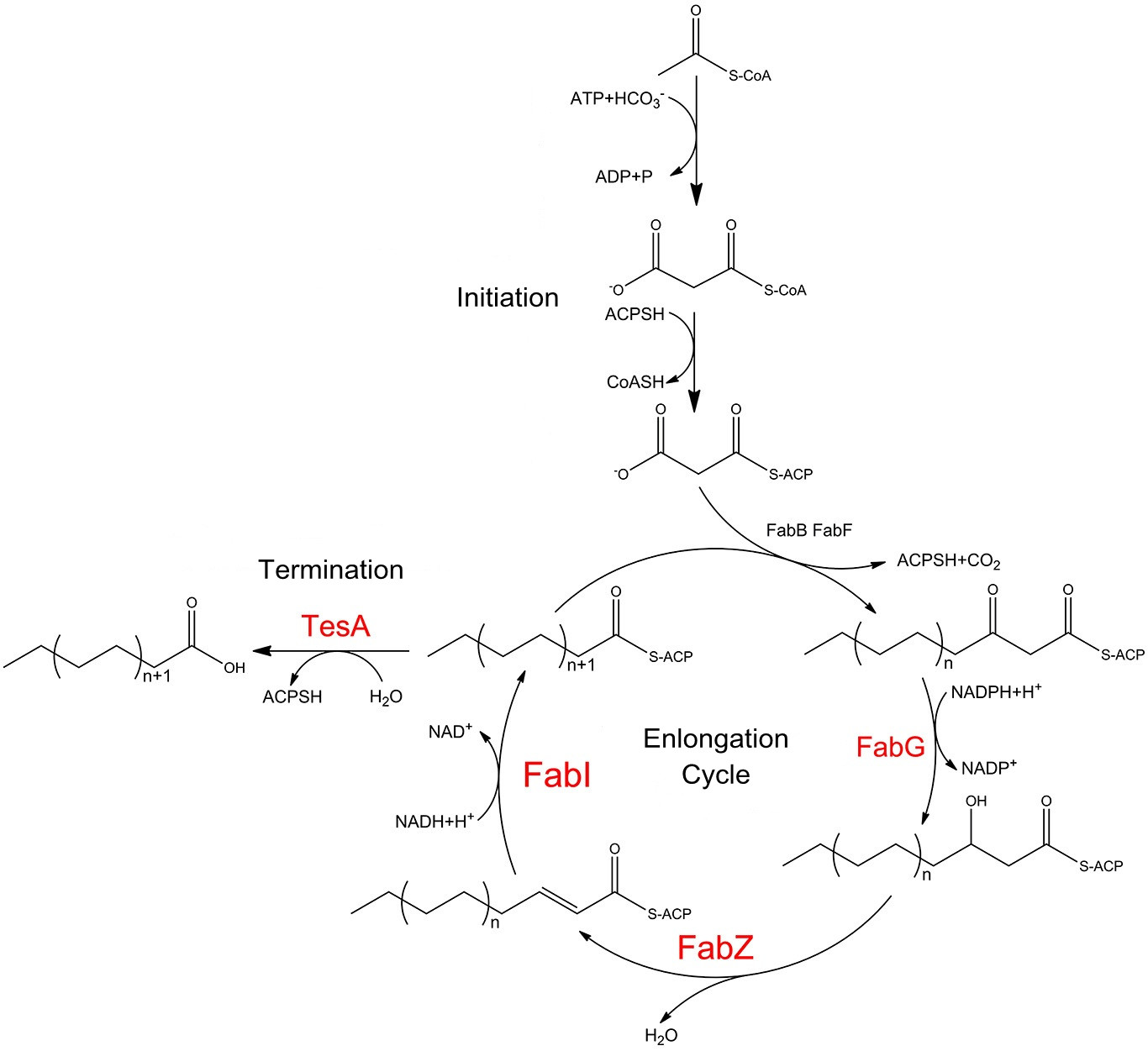
Fatty acid biosynthesis in ''E.coli'' is carried out by a nine-component enzyme system , FabA, FabB, FabD, FabF, FabG, FabH, FabI, FabZ, and ACP. They orderly cooperate to convert one equivalent of acetyl-CoA and 6-8 equivalents of malonyl-CoA into C14-C18 acyl-ACP species. The cytoplasmic mutant of the periplasmic thioesterase is capable of releasing free fatty acids preventing the fatty acyl yields from being directly harnessed for phospholipid biosynthesis. | Fatty acid biosynthesis in ''E.coli'' is carried out by a nine-component enzyme system , FabA, FabB, FabD, FabF, FabG, FabH, FabI, FabZ, and ACP. They orderly cooperate to convert one equivalent of acetyl-CoA and 6-8 equivalents of malonyl-CoA into C14-C18 acyl-ACP species. The cytoplasmic mutant of the periplasmic thioesterase is capable of releasing free fatty acids preventing the fatty acyl yields from being directly harnessed for phospholipid biosynthesis. | ||
| Line 45: | Line 47: | ||
===Design of membrane assembly=== | ===Design of membrane assembly=== | ||
| + | |||
Fusion proteins are constructed to form protein complex on the cell membrane and four enzyms are selected based on previous study. We express full complement of reductive enzymes, FabG, FabZ and FabI, which together could lead to 50% increase in fatty acid turnover and thioesterase, TesA whose moderate overexpression in ''E.coli'' gave rise to elevated fatty acid productivity. TesA are fused with transmembrane protein No.1 as stated in the Construction and FabG, FabZ and FabI with No.2 to No.4 respectively, align with corresponding reactions occurring in sequence. | Fusion proteins are constructed to form protein complex on the cell membrane and four enzyms are selected based on previous study. We express full complement of reductive enzymes, FabG, FabZ and FabI, which together could lead to 50% increase in fatty acid turnover and thioesterase, TesA whose moderate overexpression in ''E.coli'' gave rise to elevated fatty acid productivity. TesA are fused with transmembrane protein No.1 as stated in the Construction and FabG, FabZ and FabI with No.2 to No.4 respectively, align with corresponding reactions occurring in sequence. | ||
//上图 | //上图 | ||
| - | + | ||
To identify and to evaluate the advantages of the membrane scaffold system, two sets of controlled experiments were designed and conducted. | To identify and to evaluate the advantages of the membrane scaffold system, two sets of controlled experiments were designed and conducted. | ||
| Line 59: | Line 62: | ||
''M-'' protein means enzyme fused with engineered transmembrane domine localized to ''E.coli'' membrane where enzymes aggregate and cooperate. | ''M-'' protein means enzyme fused with engineered transmembrane domine localized to ''E.coli'' membrane where enzymes aggregate and cooperate. | ||
| + | |||
| + | ===Result and Discussion=== | ||
1. WT, F-TesA and M-TesA | 1. WT, F-TesA and M-TesA | ||
Revision as of 09:45, 26 September 2012
| ||
|
 "
"