Team:Paris-Saclay/Project/Notebook/Week 11
From 2012.igem.org
(Difference between revisions)
YohannPetiot (Talk | contribs) |
YohannPetiot (Talk | contribs) |
||
| Line 51: | Line 51: | ||
|- | |- | ||
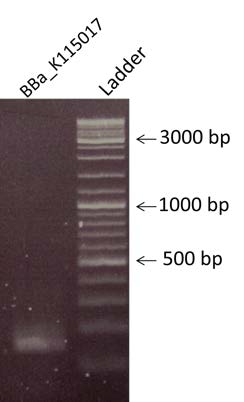
| style="width: 50%;"|Purification by PCR clean-up of BBa_K115017 treated with DPNI. To visualize the fragment, an electrophoresis has been made with a 2% Agarose gel. We are expecting a band at the size of 123 bp. | | style="width: 50%;"|Purification by PCR clean-up of BBa_K115017 treated with DPNI. To visualize the fragment, an electrophoresis has been made with a 2% Agarose gel. We are expecting a band at the size of 123 bp. | ||
| - | | style="width: 35%;"| [[File:Week11-1.jpg|right| | + | | style="width: 35%;"| [[File:Week11-1.jpg|right|280px]] |
|- | |- | ||
| - | | style="width: 50%;"|[[File:Week11-2.jpg|left| | + | | style="width: 50%;"|[[File:Week11-2.jpg|left|280px]] |
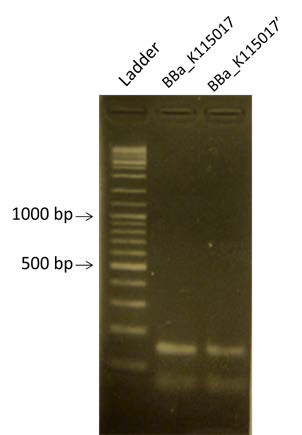
| style="width: 35%;"| New PCR of BBa_K115017 to obtain more quantity of the fragment | | style="width: 35%;"| New PCR of BBa_K115017 to obtain more quantity of the fragment | ||
|} | |} | ||
| Line 59: | Line 59: | ||
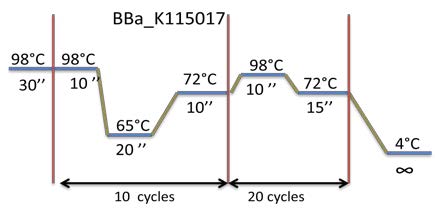
PCR program used: | PCR program used: | ||
| - | [[File:Week11-3.jpg| | + | [[File:Week11-3.jpg|500px]] |
*Digestion by DPNI of the BBa_K115017 in order to degrade the matrix plasmid. | *Digestion by DPNI of the BBa_K115017 in order to degrade the matrix plasmid. | ||
| Line 66: | Line 66: | ||
|- | |- | ||
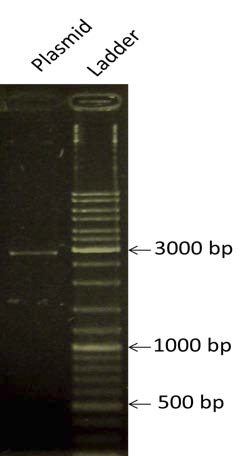
| style="width: 50%;"|Determining the concentration of the plasmid pSB1A2 which has been linearized by HINDIII. We are expecting a band at the size of (2079+935) ~3kbp | | style="width: 50%;"|Determining the concentration of the plasmid pSB1A2 which has been linearized by HINDIII. We are expecting a band at the size of (2079+935) ~3kbp | ||
| - | | style="width: 35%;"| [[File:Week11-4.jpg|right| | + | | style="width: 35%;"| [[File:Week11-4.jpg|right|280px]] |
|- | |- | ||
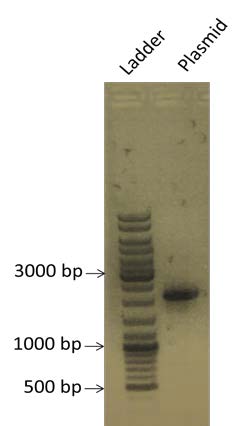
| style="width: 50%;"|A new PCR of the plasmid pSB1A2 is realized to obtain more quantity of the plasmid. We are expecting a band at the size of 2079 bp. | | style="width: 50%;"|A new PCR of the plasmid pSB1A2 is realized to obtain more quantity of the plasmid. We are expecting a band at the size of 2079 bp. | ||
| - | | style="width: 35%;"| [[File:Week11-5.jpg|right| | + | | style="width: 35%;"| [[File:Week11-5.jpg|right|280px]] |
|} | |} | ||
PCR program used: | PCR program used: | ||
| - | [[File:Week11-6.jpg| | + | [[File:Week11-6.jpg|500px]] |
| Line 81: | Line 81: | ||
|- | |- | ||
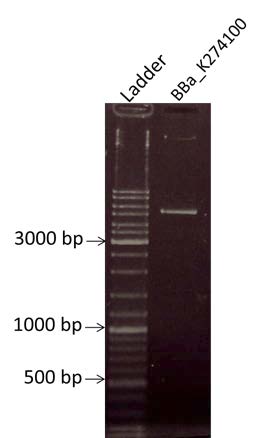
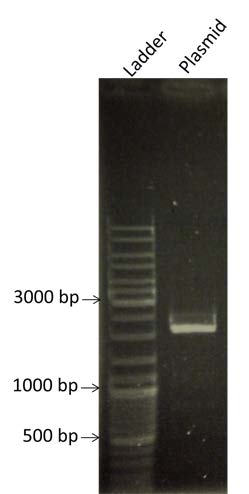
| style="width: 50%;"|Determining of the concentration of the BBa_K274100 by electrophoresis on a gel at O.8% Agarose. We are expecting a band at the size of 2100bp | | style="width: 50%;"|Determining of the concentration of the BBa_K274100 by electrophoresis on a gel at O.8% Agarose. We are expecting a band at the size of 2100bp | ||
| - | | style="width: 35%;"| [[File:Week11-7.jpg|right| | + | | style="width: 35%;"| [[File:Week11-7.jpg|right|280px]] |
|} | |} | ||
| Line 101: | Line 101: | ||
|- | |- | ||
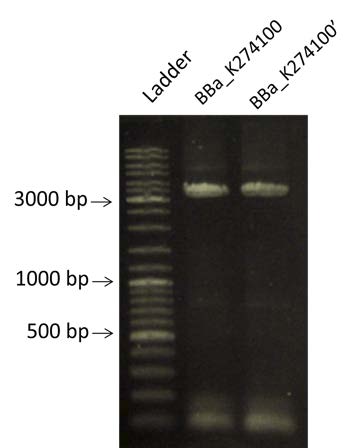
| style="width: 50%;"|PCR of BBa_K274100 and visualization by 0.8% Agarose gel electrophoresis. We are expecting a band at 3400bp. | | style="width: 50%;"|PCR of BBa_K274100 and visualization by 0.8% Agarose gel electrophoresis. We are expecting a band at 3400bp. | ||
| - | | style="width: 35%;"| [[File:Week11-8.jpg|right| | + | | style="width: 35%;"| [[File:Week11-8.jpg|right|280px]] |
|} | |} | ||
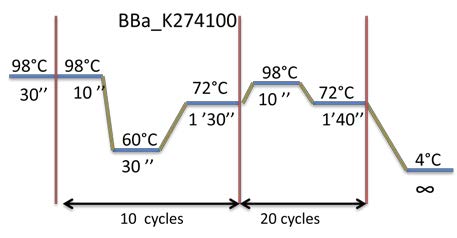
PCR program used: | PCR program used: | ||
| - | [[File:Week11-9.jpg| | + | [[File:Week11-9.jpg|500px]] |
*Digestion of the BBa_K274100 by DPNI to degrade the matrix plasmid. | *Digestion of the BBa_K274100 by DPNI to degrade the matrix plasmid. | ||
| Line 114: | Line 114: | ||
|- | |- | ||
| style="width: 50%;"|Visualization of the DPNI’s digestion of the plasmid pSB1A2 by 0.8% Agarose gel electrophoresis. . We are expecting a band at the size of 2079 bp. | | style="width: 50%;"|Visualization of the DPNI’s digestion of the plasmid pSB1A2 by 0.8% Agarose gel electrophoresis. . We are expecting a band at the size of 2079 bp. | ||
| - | | style="width: 35%;"| [[File:Week11-10.jpg|right| | + | | style="width: 35%;"| [[File:Week11-10.jpg|right|280px]] |
|} | |} | ||
Revision as of 06:26, 26 September 2012
GEMOTE

GEMOTE
13th August
| Purification by PCR clean-up of BBa_K115017 treated with DPNI. To visualize the fragment, an electrophoresis has been made with a 2% Agarose gel. We are expecting a band at the size of 123 bp. | |
| New PCR of BBa_K115017 to obtain more quantity of the fragment |
- Digestion by DPNI of the BBa_K115017 in order to degrade the matrix plasmid.
| Determining the concentration of the plasmid pSB1A2 which has been linearized by HINDIII. We are expecting a band at the size of (2079+935) ~3kbp | |
| A new PCR of the plasmid pSB1A2 is realized to obtain more quantity of the plasmid. We are expecting a band at the size of 2079 bp. |
14th August
| Determining of the concentration of the BBa_K274100 by electrophoresis on a gel at O.8% Agarose. We are expecting a band at the size of 2100bp |
- Digestion of the BBa_K274100 by DPNI to degrade the matrix plasmid.
- Stocking up cells with glycerol
- PSB1A3Amil CP + Ampicilline
- PSB1C3 Amil GFP + Chloramphinicol
15th August
Day of public holiday in France.
16th August
| PCR of BBa_K274100 and visualization by 0.8% Agarose gel electrophoresis. We are expecting a band at 3400bp. |
- Digestion of the BBa_K274100 by DPNI to degrade the matrix plasmid.
17th august
| Visualization of the DPNI’s digestion of the plasmid pSB1A2 by 0.8% Agarose gel electrophoresis. . We are expecting a band at the size of 2079 bp. |
- Miniprep of the plasmid pSB1A2.
- Miniprep of BBa_K274100 and BBa_K115017
- Gibson assembly of the B construction
- Transformation of DH5α competent cells with the Gibson B construction. The Petri dishes are placed at 37°C.
 "
"


Follow us !