Team:Wageningen UR/Coil system
From 2012.igem.org
Lisamsonne (Talk | contribs) |
Lisamsonne (Talk | contribs) |
||
| Line 57: | Line 57: | ||
After some trouble with the PCR reactions at the beginning, the correct construct was obtained, sequenced and ligated into pSB1C3 as well as BBa_J04500 (behind an IPTG induced promoter) to make it available for the Registry of Standard Biological Parts. | After some trouble with the PCR reactions at the beginning, the correct construct was obtained, sequenced and ligated into pSB1C3 as well as BBa_J04500 (behind an IPTG induced promoter) to make it available for the Registry of Standard Biological Parts. | ||
| - | Also see Journal [https://2012.igem.org/Team:Wageningen_UR/Journal/ | + | Also see Journal [https://2012.igem.org/Team:Wageningen_UR/Journal/week20 week20] (obtaining the construct)and [https://2012.igem.org/Team:Wageningen_UR/Journal/week21 week21] (transforming into the backbones) |
=="leucine zipper" coils== | =="leucine zipper" coils== | ||
Revision as of 20:02, 25 September 2012
Contents |
The Plug and Apply (PnA) system
E- and K-coil
A big challenge of our project is the attachment of ligands and functional proteins on either the outside or inside of a virus-like particle (VLP). We decided to use a noncovalent anchor-like system, which consists of two different coiled-coil proteins. For various experiments the E-/K-coil combination IAAL E3 and IAAL K3 reported by Litowksi et al. in 2002 was used.
α-helical coiled-coils represent an widely abundant but simple oligomerization motif in proteins. They have an enormous diversity of functions in nature ranging from motor proteins to transcription factors and chaperones [1]. Coiled-coils are comprised of one single secondary protein structure: the α-helix. Their quaternary structure is stable and does not unfold in aqueous solution at a neutral pH unlike other peptide α-helices. One of the striking structural features of the coiled coils is their build-up of two to five α-helices. These helices contain a heptad repeat of mainly apolar amino acids which is denoted as (abcdefg)^n in literature. The letters a and d are usually amino acids with a highly hydrophobic side chain. The side chains are packed against each other forming a hydrophobic core which allows the formation of a supercoil. Whereas the letter n is representing the number of helices in the final coil. The helices can be identical or even different in sequence and may be aligned in a parallel/antiparallel way. The amino acids of the letters e and g have typically charged residues to allow electrostatic interactions with other peptides (most importantly other coils)[2]. The process of dimerization is largely dependent on the peptide sequence and can be homodimeric (Figure 1.) or heterodimeric (e.g. E- and K-coil). In our project we are utilizing heterodimeric coiled-coils to noncovalently attach proteins. This system is advantageous because of the high stability and specificity. The use of heterodimeric coils additionally prevents the self-dimerization of either the virus capsid monomer or the protein ( or ligand) of choice.
In figure 2 the chemical interactions of a E- and K-coil pair are shown, unfortunately not the pair we have used in the course of our experiments. The wide white arrows depict the interhelical hydrophobic interactions (hydrophobic core)[2]. The thin arrows display the electrostatic attractions of the two coils on each other. As mentioned before these electrostatic interactions are caused by the amino acids present on position e and g. In this case glutamic acid (E) and lysine (K). All these interactions greatly contribute to the stability and the prevention of self-dimerization. The correct amino acid sequence of the coil pair IAAL E3 and IAAL K3, which we have implemented in our experiments is stated in table 1[2]. The choice of this pair was made to have a reasonably short coil with only 3 heptad repeats to avoid interference with the quaternary structure of the capsid proteins.
| name | peptide sequence |
| gabcdefgabcdefgabcdef | |
| IAAL E3 | Ac-EIAALEKEIAALEKEIAALEK-NH2 |
| IAAL K3 | Ac-KIAALKEKIAALKEKIAALKE -NH2 |
table 1 amino acid sequence of the chosen pair of coils (in one letter amino acid code)
GFP with E-coil
In an effort to reduce the amount of laboratory work it was decided to fuse the different capsid proteins of the VLPs with the K-coil only. We designed experiments to add the K-coil to capsid proteins of the Turnip yellows virus (TuYV), the Cowpea chlorotic mottle virus (CCMV) and to Hepatitis B (HepB) by fusion PCR (for more information see prject pages). Possible ligands and other proteins of interest are fused with the E-coil. In this way the GFP - E-coil construct for example is of universal use with either of the 3 different viruses.
Methods
Three primers where obtained to obtain this construct in 2 seperate PCR reactions fusing a E-coil on the N-terminal and a His tag on the C-terminal of GFP.
Additionally a variant without a His tag was produced using sequencing reverse primers (annealing to the backbone) instead.
Results
After some trouble with the PCR reactions at the beginning, the correct construct was obtained, sequenced and ligated into pSB1C3 as well as BBa_J04500 (behind an IPTG induced promoter) to make it available for the Registry of Standard Biological Parts.
Also see Journal week20 (obtaining the construct)and week21 (transforming into the backbones)
"leucine zipper" coils
Next to the E-/K-coil combination we are searching for suitable alternatives. We found the EILD/ KILR coils made by UC Berkeley are attractive, which are already present in the Standard parts registry of iGEM (BBa_K197021 and BBa_K197022). Both coils are GNC4 leucine zippers which can form homo- and heterodimers. The 2009 iGEM Berkeley wetlab team used this properties to show cell-cell adhesion of Escherichia coli cells, if the coils are present on the cell surface (Figure 4) [3].
Despite their proven functionality in cell-cell adhesion both coils are cloned into a BBa_197038 plasmid backbone. This backbone is very unusual for iGEM standard registry parts and is not compatible with either of the other cloning standards. Being aware of this we decided to clone the KILR-coil into a standard 10 plasmid backbone with chloramphenicol resistence to allow following iGEM teams to easily work with the Berkeley coils. This experimental approach was done only for the BBa_K197022 KILR. We designed 2 primers containing iGEM standard 10 pre- and suffix to anneal directly on the sequence of the KILR coil.
- FW primer KILR: 5’ GTTTCTTCGAATTCGCGGCCGCTTCTAGAGGATCTGTTAAAGAACTGGAAGACAAAAA 3’
- BW primer KILR: 5’ TACTAGTAGCGGCCGCTGCAGGAAGAAACCGCAACCACCACGTTCAC 3’
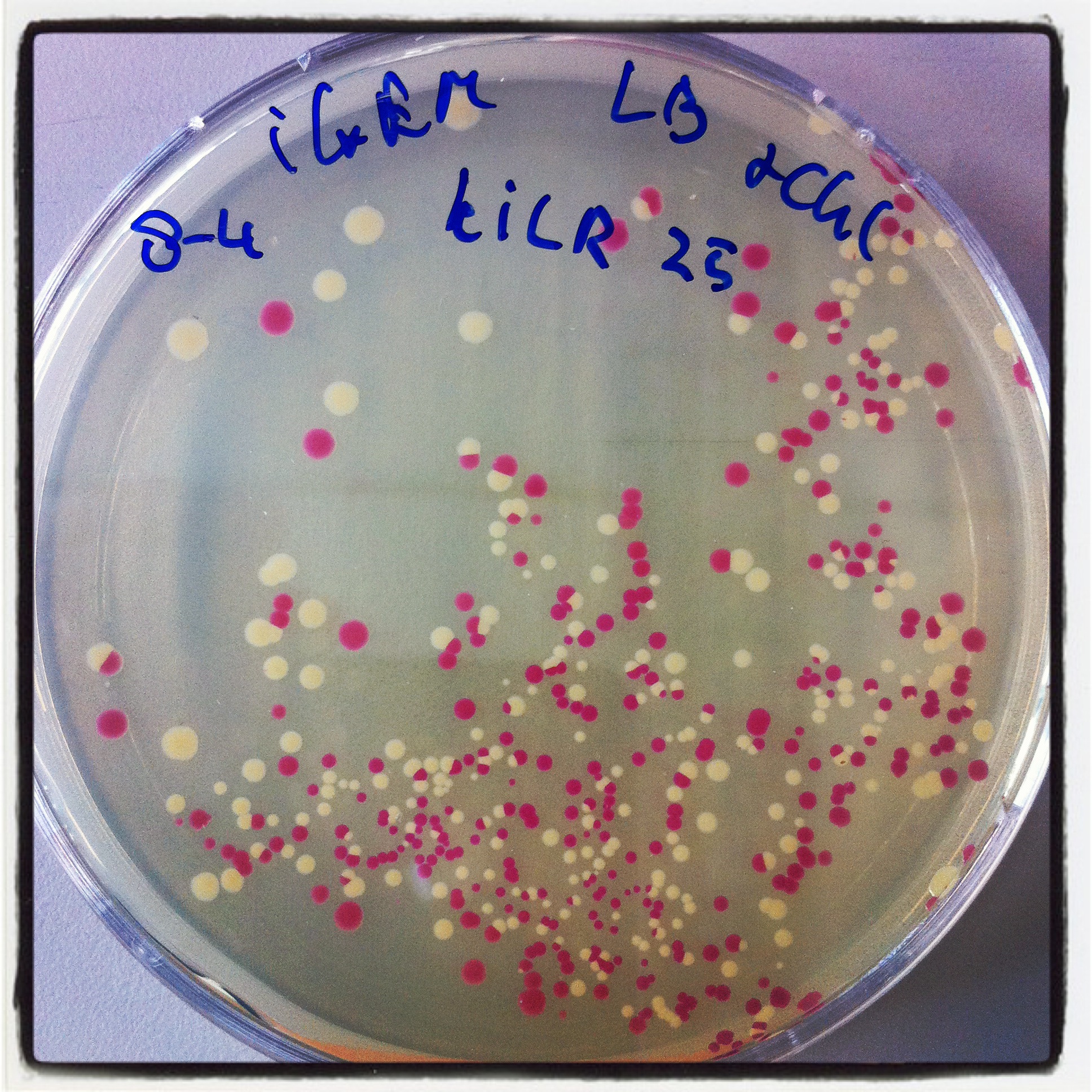
The digested PCR product was ligated into a BBa_J04450 (RFP coding device) plasmid to allow a red-white screening of the transformed colonies after transformation by electroporation.
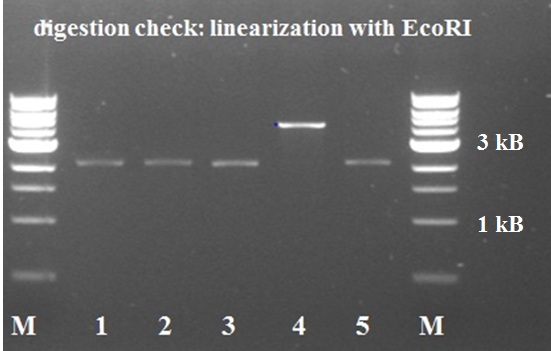
5 white colonies were miniprepped and subsequently checked by digestion with EcoRI, if they yield a plasmid of the expected size.
The gel electrophoresis of figure 7 shows the expected constructs lengths except for sample 4, which was discarded. Sample 1 and 2 were send for sequencing. Unfortunately sequencing revealed that all 2 samples lack a SpeI restriction site. But they contain the full sequence of the KILR coil (about 111bp).
Using the Parts Registry
<p align="justify"> The parts registry already contains both ligands and epitopes which can be attached to the VLPs using the PnA system. An overview is given in the table below. Many of the biobricks are still 'planning', but the design can be used to attach to the e-coil.
Table 2 Overview of epitopes and ligands from the parts registry
| Epitopes | Author | URL | Type | Status |
| V5 epitope tag tail domain (GKPIPNPLLGLDST) | Reshma Shetty | http://partsregistry.org/Part:BBa_T2018 | proteindomain/affinity | Planning |
| V5 epitope tag head domain (GKPIPNPLLGLDST) | Reshma Shetty | http://partsregistry.org/Part:BBa_T2019 | proteindomain/affinity | Planning |
| V5 epitope tag special internal domain (GKPIPNPLLGLDST) | Reshma Shetty | http://partsregistry.org/Part:BBa_T2020 | proteindomain/affinity | Planning |
| VSV-G epitope tag tail domain (YTDIEMNRLGK) | Reshma Shetty | http://partsregistry.org/Part:BBa_T2021 | proteindomain/affinity | Planning |
| VSV-G epitope tag special internal domain (YTDIEMNRLGK) | Reshma Shetty | http://partsregistry.org/Part:BBa_T2022 | proteindomain/affinity | Planning |
| ITGA4 - B epitope | QGEM_09 | http://partsregistry.org/Part:BBa_K214003 | Null | Available |
| Ligands | ||||
| Delta-mCherry Juxtacrine Signaling Ligand MammoBlock | Grant Robinson | http://partsregistry.org/Part:BBa_K511302 | Null | Planning |
| Delta-mCherry-4xFF4 Juxtacrine Signaling Ligand MammoBlock | Grant Robinson | http://partsregistry.org/Part:BBa_K511303 | Null | Planning |
| Stem cell factor (SCF) or c-kit-ligand | David Charoy | http://partsregistry.org/Part:BBa_K292009 | Null | Available |
| SH3 ligand | John Dueber | http://partsregistry.org/Part:BBa_J62002 | Null | Planning |
| SH3 ligand | John Dueber | http://partsregistry.org/Part:BBa_J62003 | Null | Planning |
- 1. Minten, I.J., et al., Controlled encapsulation of multiple proteins in virus capsids. J Am Chem Soc, 2009. 131(49): p. 17771-3.
- 2. Litowski, J.R. and R.S. Hodges, Designing heterodimeric two-stranded alpha-helical coiled-coils. Effects of hydrophobicity and alpha-helical propensity on protein folding, stability, and specificity. J Biol Chem, 2002. 277(40): p. 37272-9.
- 3. Aguado, G.G.L. Part: BBa_K197022 KILR. 2009 [09/17/2012]; Available from: http://partsregistry.org/Part:BBa_K197022.
 "
"