Team:Penn/Notebook/Biofilms
From 2012.igem.org
(Difference between revisions)
(→6/21/12) |
(→6/21/12) |
||
| Line 33: | Line 33: | ||
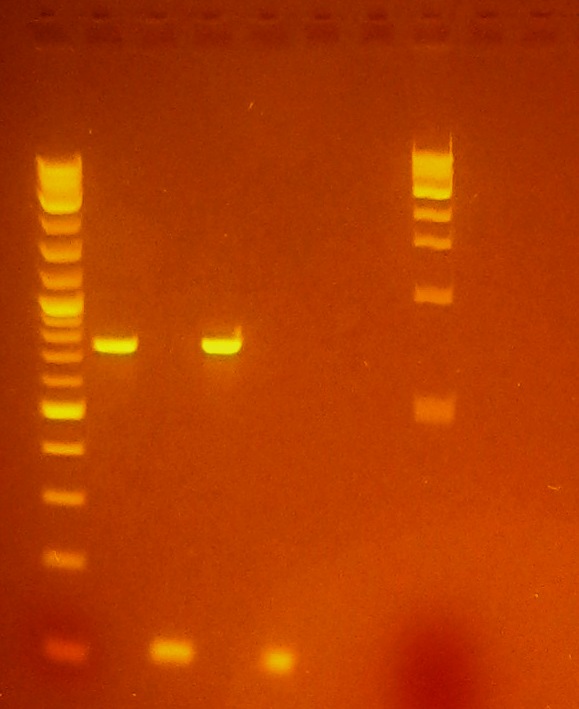
*Ran gel of yesterday's PCR purification | *Ran gel of yesterday's PCR purification | ||
[[File:PCR Purified eGFP & plsr.JPG|thumb|120px|alt=SCIENCE.|Image of 2% Agarose Gel of post-purification PCR products of eGFP (.85kb) & plsr (.1kb)]] | [[File:PCR Purified eGFP & plsr.JPG|thumb|120px|alt=SCIENCE.|Image of 2% Agarose Gel of post-purification PCR products of eGFP (.85kb) & plsr (.1kb)]] | ||
| + | **EtBr Gel 2% Agarose | ||
| + | ***Lane 1: NEB 2-log ladder (1 ug loaded) | ||
| + | ***Lane 2: PCR of eGFP using plsr_XhoI Fwd & eGFP_EcoRI Rev primers w/ 55C annealing temp. | ||
| + | ***Lane 3: PCR of plsr using plsr_BglII Fwd & plsr_XhoI Rev primers w/ 55C annealing temp. | ||
| + | ***Lane 4: PCR of eGFP using plsr_XhoI Fwd & eGFP_EcoRI Rev primers w/ 50C annealing temp. | ||
| + | ***Lane 5: PCR of plsr using plsr_BglII Fwd & plsr_XhoI Rev primers w/ 50C annealing temp. | ||
| + | ***Lane6/7: Null | ||
| + | ***Lane 8: 1kb ladder left at rt o/n | ||
*Obtained & transformed pET-26b | *Obtained & transformed pET-26b | ||
Revision as of 18:26, 21 June 2012
Contents |
6/6/12
- Set up equipment
- Autoclaved 2x 1L Sterile ddH2O
- Autoclaved 500 mL LB Broth for scale up of luxS (Plate 3 Well 2H, pSB1A2), plsr (Plate 2 Well 14H in pSB1A2 resistance to Amp)
- Added iGEM logo to wiki
6/7/12
- Resuspended available biobricks luxS & plsr (resuspended in 10uL ddH2O)
- Resuspended DNA transformed into DH5α (40uL DH5α+2uL DNA) & plated on LB plates w/ 15 uL of 100 mg/mL Amp spread on surface
- Requested lsrR & lsrK from iGEM HQ
6/8/12
- iGEM Wiki now split into two separate Notebooks
- Plenty of colonies, too many to count(TMTC) grew from plsr & luxS transformation from 6/7/12
- Selected one colony each
- Grew in 10 mL LB+Amp (100 ug/mL)
- Contacted researchers for V. harveyi bioassay & lysostaphin, V. harveyi must be purchased through ATCC ($300), lysostaphin obtained thru MTA.
6/9/12
- ~2mL of LB evaporated during incubation
- Cells spun down @ 5000 rpm for 10 min
- Miniprepped 4x Lysis into 1 column
- [plsr]=112 ng/uL
- [luxS]=114 ng/uL
6/11/12-6/16/12
- Dry lab work
6/18/12
- Obtained & resuspended primers for eGFP & plsr
6/19/12
- performed PCR w/ NEB standard taq kit w/ primers for eGFP & plsr
- Tested 2 annealing temperatures, 55C and 50C
- Ran gel w/ PCR product (2% agarose), saw clear eGFP bands, but possible .1kb obscured by loading dye.
6/20/12
- Performed qiagen PCR purification & nanodropped (it like it's hot)
6/21/12
- Ran gel of yesterday's PCR purification
- EtBr Gel 2% Agarose
- Lane 1: NEB 2-log ladder (1 ug loaded)
- Lane 2: PCR of eGFP using plsr_XhoI Fwd & eGFP_EcoRI Rev primers w/ 55C annealing temp.
- Lane 3: PCR of plsr using plsr_BglII Fwd & plsr_XhoI Rev primers w/ 55C annealing temp.
- Lane 4: PCR of eGFP using plsr_XhoI Fwd & eGFP_EcoRI Rev primers w/ 50C annealing temp.
- Lane 5: PCR of plsr using plsr_BglII Fwd & plsr_XhoI Rev primers w/ 50C annealing temp.
- Lane6/7: Null
- Lane 8: 1kb ladder left at rt o/n
- EtBr Gel 2% Agarose
- Obtained & transformed pET-26b
 "
"