Team:LMU-Munich/Weekly Journal
From 2012.igem.org
| Line 77: | Line 77: | ||
<div class="box"> | <div class="box"> | ||
'''26-31 August 2012''' | '''26-31 August 2012''' | ||
| - | + | [[Image:LMU Firstspore.jpg|200px|right]] | |
<html><a><img src="https://static.igem.org/mediawiki/2012/c/c1/SporeCoat.png" height=40"/></a></html> | <html><a><img src="https://static.igem.org/mediawiki/2012/c/c1/SporeCoat.png" height=40"/></a></html> | ||
Finally, we got our first glowing spore!! After 4 months of hard work we have our first proof that our system works.[[Image:LMU Firstspore.jpg|200px]] | Finally, we got our first glowing spore!! After 4 months of hard work we have our first proof that our system works.[[Image:LMU Firstspore.jpg|200px]] | ||
Revision as of 14:43, 8 September 2012

The LMU-Munich team is exuberantly happy about the great success at the World Championship Jamboree in Boston. Our project Beadzillus finished 4th and won the prize for the "Best Wiki" (with Slovenia) and "Best New Application Project".
[ more news ]

Weekly Journal
Legend:
| +--+--+--+--+--+-- | +--+--+--+--+-- | +--+--+--+--+--+-- | +--+--+--+--+--+-- |

|

Bacillus BioBrickBOX |

SporeCoat FusionProteins |

Germination STOP |
3-7 September 2012
 Plates of our spores diluted at 10^-2, 10^-4 and 10^-6 from the germination assay show NO GERMINATION for our triple and quadruple mutants, and plenty of germination for the WT positive control! We will try plating undiluted mutant spores to see if any germination occurs.
Plates of our spores diluted at 10^-2, 10^-4 and 10^-6 from the germination assay show NO GERMINATION for our triple and quadruple mutants, and plenty of germination for the WT positive control! We will try plating undiluted mutant spores to see if any germination occurs.
 A collection of useful tags in Freiburgs standard and with RBS included was cloned into pSB1C3. The tags are: 3xFlag, HA, cMyc, 10xHis and Streptavidin.
A collection of useful tags in Freiburgs standard and with RBS included was cloned into pSB1C3. The tags are: 3xFlag, HA, cMyc, 10xHis and Streptavidin.
26-31 August 2012
 Finally, we got our first glowing spore!! After 4 months of hard work we have our first proof that our system works.
Finally, we got our first glowing spore!! After 4 months of hard work we have our first proof that our system works.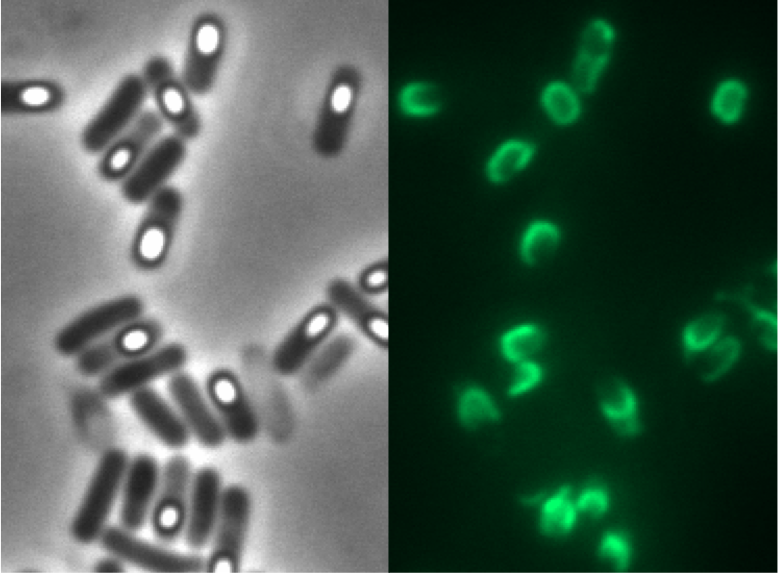
 Jara created quadruple mutants using two variations on past mutants: cwlD::kan + cwlJ::spec + gerD::cat + sleB::mls and gerD::cat + sleB::mls + cwlJ::spec + cwlD::kan.
Germination assay performed on triple and quadruple mutants.
Jara created quadruple mutants using two variations on past mutants: cwlD::kan + cwlJ::spec + gerD::cat + sleB::mls and gerD::cat + sleB::mls + cwlJ::spec + cwlD::kan.
Germination assay performed on triple and quadruple mutants.
 The BioBrick [http://partsregistry.org/wiki/index.php?title=Part:BBa_K823019 lacZ] for B. subtilis was shown to be functional in pSBBs0K-Pspac in E. coli and B. subtilis. (blue color on plates with IPTG and X-Gal)
The BioBrick [http://partsregistry.org/wiki/index.php?title=Part:BBa_K823019 lacZ] for B. subtilis was shown to be functional in pSBBs0K-Pspac in E. coli and B. subtilis. (blue color on plates with IPTG and X-Gal)
The genes [http://partsregistry.org/wiki/index.php?title=Part:BBa_K823028 luc+] and [http://partsregistry.org/wiki/index.php?title=Part:BBa_K823029 mKate2], synthesized by gene art were succesfully cloned into pSB1C3 and sequenced.
20-24 August 2012
 Double negative loop with lacZa finished -> works qualitatively (Julia)
Double negative loop with lacZa finished -> works qualitatively (Julia)
13-17 August 2012
 Jara and Jenny used last week's double mutants to create triple mutants as follows: cwlD::kan + sleB::mls + cwlJ::spec ; cwlD::kan + sleB::mls + gerD::cat ; cwlD::kan + cwlJ::spec + gerD::cat ; gerD::cat + sleB::mls + cwlJ::spec.
Jara and Jenny used last week's double mutants to create triple mutants as follows: cwlD::kan + sleB::mls + cwlJ::spec ; cwlD::kan + sleB::mls + gerD::cat ; cwlD::kan + cwlJ::spec + gerD::cat ; gerD::cat + sleB::mls + cwlJ::spec.
 ß-glactosiase assay of the Anderson promoters J23100, J23102, J23103, J23106 in pSBBs1C-lacZ in B. subtilis.
ß-glactosiase assay of the Anderson promoters J23100, J23102, J23103, J23106 in pSBBs1C-lacZ in B. subtilis.
The xylose-inducible promoter with the according repressor (which has a constitutive promoter, RBS and terminator) PXyl</> + <i>XylR was cloned into pSB1C3 and sequenced.
6-10 August 2012
 Jara and Jenny created four double-mutants using resistance-cassettes to knock out germination genes as follows: cwlD::kan + sleB::mls ; gerD::cat + sleB::mls ; gerD::cat + cwlD::kan ; cwlJ::spec + cwlD::kan. We also created the resistance cassette knockout cwlB::kan.
Jara and Jenny created four double-mutants using resistance-cassettes to knock out germination genes as follows: cwlD::kan + sleB::mls ; gerD::cat + sleB::mls ; gerD::cat + cwlD::kan ; cwlJ::spec + cwlD::kan. We also created the resistance cassette knockout cwlB::kan.
 Finally, the last PstI site could be removed and pSBBs3C-luxABCDE was completed.
Finally, the last PstI site could be removed and pSBBs3C-luxABCDE was completed.
Also, the double terminator B0014 was cloned into pSB1C3.
30 July - 3 August 2012
23-27 July 2012
 Plate reader measurements of the Bacillus promoters PliaG, PliaI and PlepA finished. Look at Data!
Plate reader measurements of the Bacillus promoters PliaG, PliaI and PlepA finished. Look at Data!
16-20 July 2012
 Plate reader measurements of the Anderson promoters J23100, J23101, J23102, J23103, J23106, J23107, J23113, J23114, J23115, J23117, J23118 in the Bacillus reporter vector pSBBs3C-luxABCDE completed. Look at Data!
Plate reader measurements of the Anderson promoters J23100, J23101, J23102, J23103, J23106, J23107, J23113, J23114, J23115, J23117, J23118 in the Bacillus reporter vector pSBBs3C-luxABCDE completed. Look at Data!
 Cloning of the Anderson promoters J23100, J23102, J23103, J23106 in the reporter vector pSBBs1C-lacZ for β-galactosidase assays finished.
Cloning of the Anderson promoters J23100, J23102, J23103, J23106 in the reporter vector pSBBs1C-lacZ for β-galactosidase assays finished.
9-13 July 2012
 The vectors pSBBs4S-PXyl , pSBBs1C-lacZ and pSBBs4S were succesfully completed and tested by restriction digest as well as red colony colour.
The vectors pSBBs4S-PXyl , pSBBs1C-lacZ and pSBBs4S were succesfully completed and tested by restriction digest as well as red colony colour.
2-6 July 2012
 Clean deletions of germination genes sleB and cwlB from PCR accomplished. DNA purified and frozen to be later transformed with Bacillus.
Clean deletions of germination genes sleB and cwlB from PCR accomplished. DNA purified and frozen to be later transformed with Bacillus.
 β-galactosidase assays of the promoters PliaG, PliaI and Pveg are finished. Look at Data!
β-galactosidase assays of the promoters PliaG, PliaI and Pveg are finished. Look at Data!
 Cloning of the Anderson promoters J23100, J23101, J23102, J23103, J23106, J23107, J23113, J23114, J23115, J23117, J23118 in pSB1C3 finished.
Cloning of the Anderson promoters J23100, J23101, J23102, J23103, J23106, J23107, J23113, J23114, J23115, J23117, J23118 in pSB1C3 finished.
25-29 June 2012
18-22 June 2012
11-15 June 2012
 Clean deletions of germination genes cwlD, cwlJ, and gerD from PCR accomplished. DNA purified and frozen to be later transformed with Bacillus.
Clean deletions of germination genes cwlD, cwlJ, and gerD from PCR accomplished. DNA purified and frozen to be later transformed with Bacillus.
4-8 June 2012
28-1 June 2012
21-25 May 2012
 Promoters PliaG, PliaI, Pveg and PlepA are now in the vector pSB1C3 as BioBrick standard for the registry. BioBricks are BBa_K823000, BBa_K823001, BBa_K823002, BBa_K823003.
Promoters PliaG, PliaI, Pveg and PlepA are now in the vector pSB1C3 as BioBrick standard for the registry. BioBricks are BBa_K823000, BBa_K823001, BBa_K823002, BBa_K823003.
 The Anderson promoters J23100, J23101, J23102, J23103, J23106, J23107, J23113, J23114, J23115, J23117, J23118 are now in the Bacillus reporter vector pSBBs3C-luxABCDE for measuring their activity as luminescence.
The Anderson promoters J23100, J23101, J23102, J23103, J23106, J23107, J23113, J23114, J23115, J23117, J23118 are now in the Bacillus reporter vector pSBBs3C-luxABCDE for measuring their activity as luminescence.
 The Bacillus promoters PliaG, PliaI and PlepA are in the Bacillus reporter vector pSBBs3C-luxABCDE.
The Bacillus promoters PliaG, PliaI and PlepA are in the Bacillus reporter vector pSBBs3C-luxABCDE.
14-18 May 2012
7-11 May 2012
 Cloning of PliaG, PliaI and Pveg in vector pSBBs1C-lacZ finished.
Cloning of PliaG, PliaI and Pveg in vector pSBBs1C-lacZ finished.
30 April-4 Mai 2012
23-27 April 2012
 pSBBs3C-luxABCDE with still one PstI site was created with RFP in the multile cloning site to have a vector for promoter measurments. edit: This PstI site was removed later and only that backbone is submitted to the registry.
pSBBs3C-luxABCDE with still one PstI site was created with RFP in the multile cloning site to have a vector for promoter measurments. edit: This PstI site was removed later and only that backbone is submitted to the registry.
16-20 April 2012
 The cloning of the reporter vector pSBBs1C-lacZ was finished.
The cloning of the reporter vector pSBBs1C-lacZ was finished.
9-13 April 2012
 Jara created the single mutant cwlD::kan.
Jara created the single mutant cwlD::kan.
2-6 April 2012
 Jara successfully created our first single knockouts of germination genes using a resistance cassettes: sleB::mls, gerD::cat and cwlJ::spec.
Jara successfully created our first single knockouts of germination genes using a resistance cassettes: sleB::mls, gerD::cat and cwlJ::spec.
Jara tried knocking out cwlB using the kan resistance cassette. Mutants of cwlB::kan grew very poorly.
 With pSBBs0K-Pspac our first Vector for our Bacillus BioBrick Box was completed.
With pSBBs0K-Pspac our first Vector for our Bacillus BioBrick Box was completed.
26-30 March 2012
 Jara tried knocking out cwlB using the tet resistance cassette. Mutants of cwlB::tet grew very poorly.
Jara tried knocking out cwlB using the tet resistance cassette. Mutants of cwlB::tet grew very poorly.
19-23 March 2012
 We decided which genes to knock out for the germination stop. Based on the work of [http://www.ncbi.nlm.nih.gov/pubmed/19554258 J. Kim and W. Schumann (2009)], we decided to knock out genes cwlB, gerD, cwlJ, and sleB. From the research of [http://www.ncbi.nlm.nih.gov/pubmed/11466293 B. Setlow et al (2001)], we also chose cwlD.
We decided which genes to knock out for the germination stop. Based on the work of [http://www.ncbi.nlm.nih.gov/pubmed/19554258 J. Kim and W. Schumann (2009)], we decided to knock out genes cwlB, gerD, cwlJ, and sleB. From the research of [http://www.ncbi.nlm.nih.gov/pubmed/11466293 B. Setlow et al (2001)], we also chose cwlD.
12-16 March 2012
5-9 March 2012
27 February - 2 March 2012
20-24 February 2012
Team fully formed!
Antibiotic abbreviation legend:
cat: chloramphenicol
kan: kanamycin
mls: Macrolide-Lincosamide-Streptogramin B
spec: spectinomycin
tet: tetracycline
 "
"



