Team:Chalmers-Gothenburg/Theory
From 2012.igem.org
m (→G protein-coupled receptors) |
m |
||
| (55 intermediate revisions not shown) | |||
| Line 1: | Line 1: | ||
{{Team:Chalmers-Gothenburg/Template2}} | {{Team:Chalmers-Gothenburg/Template2}} | ||
| - | <div align="justify" style="width:auto; margin-left:0px; margin-right: | + | <html> |
| - | ==Introduction== | + | <head> |
| + | <style type="text/css"> | ||
| + | div.box2 | ||
| + | { | ||
| + | width:250px; | ||
| + | height:600px; | ||
| + | background-color:#FFFFFF; | ||
| + | border:0px solid #D8D8D8; | ||
| + | } | ||
| + | |||
| + | div.box2 p | ||
| + | { | ||
| + | margin:20px 20px; | ||
| + | font-weight:normal; | ||
| + | color:#000000; | ||
| + | align:justify | ||
| + | } | ||
| + | |||
| + | </style> | ||
| + | </head> | ||
| + | |||
| + | <body> | ||
| + | |||
| + | <div class="box2"; style="position:absolute;left:700px;top:330px;"> | ||
| + | <p><div align="left" style="margin-left:10px; margin-right:10px; "> <b><font size="9"><b>Sponsors</b></font></b> | ||
| + | </br> | ||
| + | </br> | ||
| + | </br> | ||
| + | <a href="http://microsynth.ch/" target="_blank"> | ||
| + | <img src="https://static.igem.org/mediawiki/2012/7/74/Microsynth_Logo_color.jpg" width="220" alt="Microsynth" /></a> | ||
| + | </br> | ||
| + | </br> | ||
| + | </br> | ||
| + | <a href="http://www.gatc-biotech.com/en/index.html" target="_blank"> | ||
| + | <img src="https://static.igem.org/mediawiki/2012/c/c1/GATC.gif" width="220" alt="GATC Biotech" /></a> | ||
| + | </br> | ||
| + | </br> | ||
| + | </br> | ||
| + | <a href="http://www.chalmers.se/en/Pages/default.aspx" target="_blank"> | ||
| + | <img src="https://static.igem.org/mediawiki/2012/d/dc/Chalmers40.png" width="150" alt="Chalmers" /></a> | ||
| + | </br> | ||
| + | </br> | ||
| + | </br> | ||
| + | <a href="http://www.molnlycke.com/com/" target="_blank"> | ||
| + | <img src="https://static.igem.org/mediawiki/2012/6/69/Molnlycke.jpg" width="220" alt="Mölnlycke Health Care" /></a> | ||
| + | |||
| + | |||
| + | </p> | ||
| + | </p> | ||
| + | </div> | ||
| + | </div> | ||
| + | </body> | ||
| + | |||
| + | </html> | ||
| + | <div align="justify" style="width:auto; margin-left:0px; margin-right:300px;"> | ||
| + | __NOEDITSECTION__ | ||
| + | |||
| + | |||
| + | =='''Introduction'''== | ||
In this section we present some information needed to better understand the design of our biosensor. | In this section we present some information needed to better understand the design of our biosensor. | ||
Firstly, a short introduction to G protein-coupled receptors will be given. Thereafter, the hCG hormone, its associated LH/CG receptor and their roles during pregnancy will be discussed. The construction of | Firstly, a short introduction to G protein-coupled receptors will be given. Thereafter, the hCG hormone, its associated LH/CG receptor and their roles during pregnancy will be discussed. The construction of | ||
| Line 9: | Line 67: | ||
approaches within these areas. | approaches within these areas. | ||
| - | == G protein-coupled receptors == | + | =='''G protein-coupled receptors'''== |
| - | In our biosensor we will utilize the LH/CG receptor which is a member of the family of G protein-coupled receptors (GPCRS). GPCRs are the largest class of cell-surface receptors and are used by all eukaryotic organisms, including yeast. They mediate responses to a wide variety of extracellular signals such as smell, taste, light, neurotransmitters and hormones [[#TheCell|[1]]] and they control important functions like heart rate and blood pressure. GPCRs are also the most common target for medicinal drugs today and nearly 30 % of all pharmaceuticals function by acting as modulators of GPCRs [[#Ishii10|[2]]]. | + | In our biosensor we will utilize the LH/CG receptor, which is a member of the family of G protein-coupled receptors (GPCRS). GPCRs are the largest class of cell-surface receptors and are used by all eukaryotic organisms, including yeast. They mediate responses to a wide variety of extracellular signals such as smell, taste, light, neurotransmitters and hormones [[#TheCell|[1]]] and they control important functions like heart rate and blood pressure. GPCRs are also the most common target for medicinal drugs today and nearly 30 % of all pharmaceuticals function by acting as modulators of GPCRs [[#Ishii10|[2]]]. |
| - | + | ====Structure==== | |
| - | All GPCRs are made up of a single peptide with a cytosolic region, a ligand-binding extracellular region and seven transmembrane domains. The transmembrane domains are connected via three intracellular and three extracellular loops. Common for | + | All GPCRs are made up of a single peptide with a cytosolic region, a ligand-binding extracellular region and seven transmembrane domains. The transmembrane domains are connected via three intracellular and three extracellular loops. Common for GPCRs is also that they mediate responses to extracellular signals by interacting and activating a trimeric GTP-binding protein (G protein). All G proteins have a similar structure and consist of three subunits called α, β and γ. These are in turn divided into several subclasses. For instance, there are more than 15 identified Gα subunits, which are sorted into four main classes: Gαi/o, Gαs, Gαq and Gα12 [[#Brown00|[3]]]. |
| - | + | ====Signal transduction==== | |
| - | The activation of the G protein is achieved by a conformational change of the receptor after binding of a signal molecule to | + | The activation of the G protein is achieved by a conformational change of the receptor after binding of a signal molecule to its ligand binding site. This change causes the α subunit to release its bound GDP and to exchange it for GTP. This in turn leads to a conformational change of the G protein itself, which is thereby activated. At this stage the subunits of the G protein can either dissociate or remain associated. In either case, the activated G protein then interacts with a target protein in the cell membrane, which relays the signal [[#TheCell|[1]]]. The figure below shows a schematic illustration of a G protein-coupled receptor and its interacting G protein. |
| - | [[File: | + | [[File:Gpcr.png|center|650px]] |
| - | <p align="justify"; style="width:auto; margin-left: | + | <p align="justify"; style="width:auto; margin-left:50px; margin-right:50px; font-size:11.5px;">'''Schematic illustrations of a G protein-coupled receptor (GPCR).''' The GPCR consists of a cytosolic region, a ligand-binding extracellular region and seven transmembrane domains. The receptor is associated with a trimeric GTP-binding protein (G protein) consisting of three subunits called α, β and γ. '''(A)''' When no signal molecule is present, the G protein binds to GDP and is inactive. '''(B)''' Upon binding of a signal protein to the ligand-binding site, a conformational change of the receptor occurs. This enables the receptor to interact with the α subunit of the associated G protein, which then exchanges its bound GDP to GTP. This activates the G protein and causes the βγ subunit to dissociate, thus enabling it to relay the signal by regulating the activity of additional intracellular signaling proteins. Figure is adapted from [[#TheCell|[1]]].</p> |
| - | == Human chorionic gonadotropin hormone== | + | =='''Human chorionic gonadotropin hormone'''== |
| + | [[File:Hcglevels.jpg|thumb|'''hCG levels during pregnancy'''. Concentration of total hCG in urine from pregnant women. [[#Cole10|[6]]] ]] [[File:Alphabeta.jpg|thumb|'''Crystal structure of hCG'''. The hormone consist of an α and a β subunit.[[#RCSB|[7]]] ]] | ||
The hormone of interest when detecting pregnancy is human chorionic gonadotropin (hCG), which is a hormone that belongs to the family of glycoproteins [[#Rendeva|[4]]]. During pregnancy, hCG will bind to the luteinizing hormone receptor (LH/CG receptor) that is localized on the yellow body and ensure the yellow body to maintains its progesterone production. This maintained level of progesterone stops menstrual bleeding and prepares the uterus lining for the implementation of the fertilized egg [[#Racowsky|[5]]], [[#Cole10|[6]]]. | The hormone of interest when detecting pregnancy is human chorionic gonadotropin (hCG), which is a hormone that belongs to the family of glycoproteins [[#Rendeva|[4]]]. During pregnancy, hCG will bind to the luteinizing hormone receptor (LH/CG receptor) that is localized on the yellow body and ensure the yellow body to maintains its progesterone production. This maintained level of progesterone stops menstrual bleeding and prepares the uterus lining for the implementation of the fertilized egg [[#Racowsky|[5]]], [[#Cole10|[6]]]. | ||
| - | All glycoprotein hormones share a common α subunit, but they also consist of a receptor-specifc β subunit. The hCG hormone is made up of an α and a β subunit that consist of 92 respectively 145 amino acid residues [[#Cole|[ | + | All glycoprotein hormones share a common α subunit, but they also consist of a receptor-specifc β subunit. The hCG hormone is made up of an α and a β subunit that consist of 92 respectively 145 amino acid residues [[#Cole|[8]]]. The term hCG actually refers to five independent molecules, all with various structure and biological function. The two dominating forms are regular hCG and hyperglycosylated hCG (H-hCG) but only regular hCG acts on the LH/CG receptor. Therefore our biosensor will only be able to detect regular hCG [[#Cole|[8]]]. |
| - | |||
| - | |||
| - | |||
| - | + | =='''Luteinizing hormone receptor'''== | |
| - | + | The LH/CG receptor belongs to the family of G proteins-coupled receptors [[#Ryu|[9]]] and it is the receptor that we will utilize when constructing our biosensor. During pregnancy, the LH/CG receptor regulates levels of progesterone promoting growth of the fetus. The receptor is located in fetal organs and in fallopian and uterine tissues in women. It can also be found in regions of the adult brain such as hippocampus, hypothalamus and brain stem [[#Cole10|[6]]]. | |
| - | + | ====Structure==== | |
| - | + | The extracellular domain is responsible for binding hCG and is characterized by a number of leucine-rich repeats. The transmembrane domains are needed for receptor activation and signal transduction after binding of the hormone. In addition, intracellular loop III is most likely physically linked to the corresponding G protein of the receptor [[#Ryu2|[10]]]. The extracellular domain of the LH/CG receptor also contains six potential sites for glycosylation. Experiments have shown that the deglycoslyated LH/CG receptor binds native hCG with the same affinity as the glycosylated receptor and is able to activate the intracellular pathway. However, some of the glycosylation sites of the receptor may affect intracellular transport, membrane insertion or recycling of the receptor [[#Petaja|[11]]]. | |
| - | Saccharomyces cerevisiae (hereafter referred to as "yeast") is the most investigated eukaryotic organism and it is the host that we will utilize when expressing our human GPCR in this project. Mammalian and yeast GPCRs are structurally and functionally very similar and several heterologous GPCRs have successfully been expressed in yeast [[#Ishii10|[2]]]. Because of this and due to its acceptability to genetic modifications, yeast is an attractive host for studying GPCRs [[#Ladds|[ | + | ====Activation and signal transduction==== |
| + | |||
| + | Researches indicates that hCG has an unique way to trigger its receptor. Initially, hCG binds to the extracellular domain of the LH/CG receptor which leads to an conformational change. This enables a secondary binding that activates the receptor and the signal is transduced through the transmembrane domains. This results in a conformational change of the intracellular loops and cytoplasmic C-terminal tail, which in turn activates the α subunit of a G protein, commonly Gαs or to varying degrees Gαq and Gαi [[#Dufau|[12]]]. Activation of Gα then results in the activation of the cAMP-dependent pathway [[#Ryu|[9]]]. | ||
| + | |||
| + | =='''Yeast as a model organism in the study of GPCRs'''== | ||
| + | |||
| + | ''Saccharomyces cerevisiae'' (hereafter referred to as "yeast") is the most investigated eukaryotic organism and it is the host that we will utilize when expressing our human GPCR in this project. Mammalian and yeast GPCRs are structurally and functionally very similar and several heterologous GPCRs have successfully been expressed in yeast [[#Ishii10|[2]]]. Because of this and due to its acceptability to genetic modifications, yeast is an attractive host for studying GPCRs [[#Ladds|[13]]]. In addition, the yeast pheromone response pathway, which is activated by endogenous yeast GPCRs, is very well-studied and has proven to bear a significant resemblance to mammalian GPCR signaling [[#Erlenbach|[14]]]. By exploiting the fact that several of the heterologously expressed GPCRs can be coupled with the pheromone pathway and by the use of different reporter genes, the yeast provides a simple tool for analysis of the GPCRs [[#RyoS|[15]]]. [[#Yeast pheromone pathway|Here]] you can read more about the pheromone pathway. | ||
Yeast-based expression of GPCRs does, however, have some limitations. Two of these, which we have identified as being of importance for this project, are discussed below. | Yeast-based expression of GPCRs does, however, have some limitations. Two of these, which we have identified as being of importance for this project, are discussed below. | ||
| - | + | ====The yeast cell wall==== | |
| - | The cell wall of yeast can pose a problem in some situations when studying GPCRs | + | The cell wall of yeast can pose a problem in some situations when studying GPCRs. Apart from stabilizing the yeast cell, the cell wall also serves as a scaffold for external glycoproteins. These glycoproteins and especially their carbohydrate side-chains limit the permeability of the cell wall [[#Klis|[16]]] and large molecules (Mr > 5000 Dalton) will not easily pass through it [[#Ladds|[13]]]. Hence, if a ligand of a heterologously expressed GPCR is larger than about 70-80 amino acid residues, there is risk that it will not be able to activate the receptor [[#Ladds|[13]]]. The hCG hormone has two subunits consisting of 92 and 145 amino acids respectively [[#Cole|[8]]] and the size of the hormone could thus constitute a problem for the hCG biosensor. Removing the cell wall with enzymes could be a solution but it is impractical and often results in less viable yeast cells [[#Ladds|[13]]]. In our [https://2012.igem.org/Team:Chalmers-Gothenburg/Methods Methods] section you can read about how we are planning to overcome this problem. |
| - | + | ====Glycosylation in yeast==== | |
| - | Glycosylation is the most common post-translational modification of GPCRs [[#Yurugi|[ | + | Glycosylation is the most common post-translational modification of GPCRs [[#Yurugi|[17]]] and approximately 95 % of all GPCRs are glycosylated [[#Lanctot|[18]]]. Another possible limitation when using yeast to study GPCRs is that mammalian GPCRs expressed in yeast will be glycosylated in a significantly different way than when expressed in a mammalian cell [[#Hamilton|[19]]]. There have also been cases of heterologous GPCRs not being glycosylated at all when expressed in yeast [[#Gilchrist|[20]]]. Thus, problems may arise when expressing heterologous GPCRs in yeast that require proper glycosylation for the correct ligand-binding and signal-transduction [[#Gilchrist|[20]]]. The LH/CG receptor, the GPCR used in this project, has a number of glycosylated sites and we have yet to see how and if the receptor will function without these when being expressed in yeast. However, according to Petäja-Repo et al. 1993 [[#Petaja|[11]]], glycosylation is required for neither the binding of the hCG hormone to the LH/CG receptor nor the correct signal transduction. On the other hand, its glycosylation might be important for the receptor to be correctly transported to and integrated in the cell membrane [[#Petaja|[11]]]. |
| - | == Yeast pheromone pathway == | + | =='''Yeast pheromone pathway'''== |
| - | S.cerevisiae stably exists as either a haploid or a diploid. In both modes of existence, it reproduces asexually through a process called budding. This is an asymmetrical dividing process in which a mother cell gives rise to a smaller daughter cell [[#Feldmann|[ | + | ''S.cerevisiae'' stably exists as either a haploid or a diploid. In both modes of existence, it reproduces asexually through a process called budding. This is an asymmetrical dividing process in which a mother cell gives rise to a smaller daughter cell [[#Feldmann|[21]]]. Diploid yeast cells are stable but under stress conditions such as absence of nutrition, they can form haploid spores. In the haploid mode, yeast has two mating types, a and α (alpha) called MATa and MATα(alpha) respectively. The haploid yeast cells are capable to mate with the opposite mating type which results in the formation of a diploid zygote [[#Bardwell|[22]]]. |
| - | Mating between haploid cells is mediated by diffusible molecules called pheromones. The mating types secrete different pheromones (a- and α-factors) and respond to the mating factor of the opposite mating type. The response occurs when the pheromones bind to a G protein-coupled receptor in the yeast cell membrane. The two mating types have different GPCRs (Ste2 and Ste3) each binding to the corresponding pheromone. The pheromones activate the GPCR which in turn creates an intracellular response. This intracellular signal transduction pathway in yeast is one the best understood signaling pathways in eukaryotes [[# | + | Mating between haploid cells is mediated by diffusible molecules called pheromones. The mating types secrete different pheromones (a- and α(alpha)-factors) and respond to the mating factor of the opposite mating type. The response occurs when the pheromones bind to a G protein-coupled receptor in the yeast cell membrane. The two mating types have different GPCRs (Ste2 and Ste3) each binding to the corresponding pheromone. The pheromones activate the GPCR which in turn creates an intracellular response. This intracellular signal transduction pathway in yeast is one the best understood signaling pathways in eukaryotes [[#Ishii10|[2]]] and will also be utilized in the hCG biosensor. The whole pathway is illustrated in the Figure below. |
| - | + | ====Signal transduction==== | |
| - | + | ||
| + | The GPCR of the MATa mating type is Ste2. Its associated G protein at the inside of the cell membrane consists of three subunits, Gpa1, Ste4 and Ste18, which correspond to mammalian Gα, Gβ and Gγ [[#Ishii10|[2]]]. When α(alpha)-factors from the MATα(alpha) cell bind to the Ste2 receptor of the MATa-cell, a conformational change of the GPCR occurs. This activates the α(alpha) subunit of the G protein, the Gpa1, by exchanging its bound GDP for GTP. This leads to the dissociation of the Ste4-Ste18 complex, which in turn binds the effector proteins Ste5 and Ste20, through Ste4. Ste20 is a protein kinase and Ste5 is a scaffold protein that binds and organizes different kinases of the mitogen-activated protein kinase (MAPK) cascade. When the Ste4-Ste18 complex binds to both Ste5 and Ste20, the two proteins are moved towards each other. This enables Ste20 to phosphorylate the first kinase in the MAPK cascade, Ste11, which is bound to Ste5. Consequently, subsequent kinases (Ste7 and Fus3) of the cascade are phosphorylated, which ultimately results in the phosphorylation and activation Far1 and Ste12 [[#Bardwell|[22]]]. | ||
| - | [[File:Pathway.jpg|center|frame|'''Overview of the pheromone pathway in yeast.''' (A) In absence of pheromones, the G protein, consisting of Gpa1, Ste4 and Ste18, is inactive and can therefore not mobilize the components of the signaling pathway. (B) When a pheromone molecule binds to Ste2, the conformation of the receptor changes which leads to the exchange of GDP to GTP in Gpa1. This results in the dissociation of the Ste4-Ste18 complex. Ste4 can then bind to Ste20, a protein kinase, and to the scaffold protein Ste5, which organizes the kinases of the mitogen-activated protein kinase (MAPK) pathway. Ste20 phosphorylates the first kinase in the cascade (Ste11), which leads the phosphorylation of the subsequent kinases in the pathway (Ste7 and Fus3). Finally, Ste12, a transcription factor, is phosphorylated and transcription of pheromone-induced genes occurs. Another eject of the pathway is the phosphorylation of Far1 which results in cell cycle arrest. Sst2 is a negative feedback regulator and stimulates the hydrolysis of GTP in order to inactivate the signaling cascade. Figure is adapted from [[# | + | [[File:Pathway.jpg|center|650px]] |
| + | <p align="justify"; style="width:auto; margin-left:120px; margin-right:120px; font-size:11.5px;">'''Overview of the pheromone pathway in yeast.''' '''(A)''' In absence of pheromones, the G protein, consisting of Gpa1, Ste4 and Ste18, is inactive and can therefore not mobilize the components of the signaling pathway. '''(B)''' When a pheromone molecule binds to Ste2, the conformation of the receptor changes which leads to the exchange of GDP to GTP in Gpa1. This results in the dissociation of the Ste4-Ste18 complex. Ste4 can then bind to Ste20, a protein kinase, and to the scaffold protein Ste5, which organizes the kinases of the mitogen-activated protein kinase (MAPK) pathway. Ste20 phosphorylates the first kinase in the cascade (Ste11), which leads the phosphorylation of the subsequent kinases in the pathway (Ste7 and Fus3). Finally, Ste12, a transcription factor, is phosphorylated and transcription of pheromone-induced genes occurs. Another eject of the pathway is the phosphorylation of Far1 which results in cell cycle arrest. Sst2 is a negative feedback regulator and stimulates the hydrolysis of GTP in order to inactivate the signaling cascade. Figure is adapted from [[#Ishii10|[2]]].</p> | ||
| + | |||
| + | |||
| + | <!--[[File:Pathway.jpg|center|frame|'''Overview of the pheromone pathway in yeast.''' '''(A)''' In absence of pheromones, the G protein, consisting of Gpa1, Ste4 and Ste18, is inactive and can therefore not mobilize the components of the signaling pathway. '''(B)''' When a pheromone molecule binds to Ste2, the conformation of the receptor changes which leads to the exchange of GDP to GTP in Gpa1. This results in the dissociation of the Ste4-Ste18 complex. Ste4 can then bind to Ste20, a protein kinase, and to the scaffold protein Ste5, which organizes the kinases of the mitogen-activated protein kinase (MAPK) pathway. Ste20 phosphorylates the first kinase in the cascade (Ste11), which leads the phosphorylation of the subsequent kinases in the pathway (Ste7 and Fus3). Finally, Ste12, a transcription factor, is phosphorylated and transcription of pheromone-induced genes occurs. Another eject of the pathway is the phosphorylation of Far1 which results in cell cycle arrest. Sst2 is a negative feedback regulator and stimulates the hydrolysis of GTP in order to inactivate the signaling cascade. Figure is adapted from [[#Ishii10|[2]]].]]--> | ||
<!-- | <!-- | ||
| Line 94: | Line 160: | ||
--> | --> | ||
| - | + | =='''Indigo'''== | |
| - | ==Indigo== | + | Bio-indigo can be synthesized from the amino acid tryptophan in a few simple steps, which are outlined in the picture below. The first reaction is the conversion of tryptophan to indole, catalyzed by tryptophanase. The next part of the pathway is catalyzed by a mono- or dioxygenase and transforms indole to indoxyl. Indoxyl is then converted to indigo by several spontaneous reactions in the presence of oxygen [[#Han|[23]]], [[#Berry|[24]]]. In order to produce bio-indigo in ''S. cerevisiae'', genes encoding tryptophanase and a flavin-containing monooxygenase are inserted. |
| - | Bio-indigo can be synthesized from the amino acid tryptophan in a few simple steps, which are outlined in the picture below. The first reaction is the conversion of tryptophan to indole, catalyzed by tryptophanase. The next part of the pathway is catalyzed by a mono- or dioxygenase and transforms indole to indoxyl. Indoxyl is then converted to indigo by several spontaneous reactions in the presence of oxygen [[#Han|[ | + | |
[[File: Tryptophan.png|center|frame| '''Conversion of tryptophan to indigo.''' Two enzymes, tryptophanase and mono- or dioxygenase, are needed to catalyze the reactions from tryptophan to indoxyl. The conversion from indoxyl to | [[File: Tryptophan.png|center|frame| '''Conversion of tryptophan to indigo.''' Two enzymes, tryptophanase and mono- or dioxygenase, are needed to catalyze the reactions from tryptophan to indoxyl. The conversion from indoxyl to | ||
| - | indigo occurs spontaneously in the presence of oxygen. Figure is adapted from [[#Han|[ | + | indigo occurs spontaneously in the presence of oxygen. Figure is adapted from [[#Han|[23]]], [[#Berry|[24]]].]] |
</div> | </div> | ||
| Line 112: | Line 177: | ||
<div id="Brown00"></div> [3] Brown AJ, Dyos SL, Whiteway MS, White JHM, Watson MAEA, Marzioch M, et al. Functional coupling of mammalian receptors to the yeast mating pathway using novel yeast/mammalian G protein α-subunit chimeras. Yeast. 2000;16(1):11-22. | <div id="Brown00"></div> [3] Brown AJ, Dyos SL, Whiteway MS, White JHM, Watson MAEA, Marzioch M, et al. Functional coupling of mammalian receptors to the yeast mating pathway using novel yeast/mammalian G protein α-subunit chimeras. Yeast. 2000;16(1):11-22. | ||
| - | <div id="Rendeva"></div> [4] [http://search.proquest.com/docview/207445721] Randeva HS, Jackson A, Karteris E, Hillhouse EW. hCG production and activity during pregnancy. Fetal and Maternal Medicine Review. 2001;12(3):191-208. | + | <div id="Rendeva"></div> [4] <!--[http://search.proquest.com/docview/207445721]--> Randeva HS, Jackson A, Karteris E, Hillhouse EW. hCG production and activity during pregnancy. Fetal and Maternal Medicine Review. 2001;12(3):191-208. |
| - | <div id="Racowsky"></div> [5] [http://www.sciencedirect.com/science/article/pii/S1569258298800962] Racowsky C, Gelety TJ. The Biology of the ovary. In: Bittar EE, Bittar NE, editors. Reproductive Endocrinology and Biology vol.12. Madison; 1998. p. 77-102. | + | <div id="Racowsky"></div> [5] <!--[http://www.sciencedirect.com/science/article/pii/S1569258298800962]--> Racowsky C, Gelety TJ. The Biology of the ovary. In: Bittar EE, Bittar NE, editors. Reproductive Endocrinology and Biology vol.12. Madison; 1998. p. 77-102. |
<div id="Cole10"></div> [6] Cole LA. Biological functions of hCG and hCG-related molecules. Reproductive biology and endocrinology. 2010;8(1):102-116. | <div id="Cole10"></div> [6] Cole LA. Biological functions of hCG and hCG-related molecules. Reproductive biology and endocrinology. 2010;8(1):102-116. | ||
| - | <div id=" | + | <div id="RCSB"></div> [7] Crystal Structure of Human Chorionic Gonadotropin [Online]. RCSB Protein Data Bank. Available from http://www.rcsb.org/pdb/explore/explore.do?structureId=1HRP. |
| + | |||
| + | <div id="Cole"></div> [8] <!--[http://www.sciencedirect.com/science/article/pii/S0009898111005456]--> Cole LA. hCG, Five independent molecules. Clinica chimica acta; international journal of clinical chemistry. 2012;411(1-2):48-65. | ||
| + | |||
| + | <div id="Ryu"></div> [9] <!-- [http://ac.els-cdn.com/S0020729298800015/1-s2.0-S0020729298800015-main.pdf?_tid=505e93f11a37adb80e9e59d45413eb17&acdnat=1341401833_c0dd0020052557a52938e26009a15ae7]--> Ryu KS, Gilchrist RL, Koo YB, Ji I, Ji TH. Gene, interaction, signal generation, signal divergence and signal transduction of the LH/CG recptor. International Journal of Gynecology & Obsterics. 1998;60(1):9-20. | ||
| + | |||
| + | <div id="Ryu2"></div> [10] <!--[http://ac.els-cdn.com/S0303720796039512/1-s2.0-S0303720796039512-main.pdf?_tid=0ab27504e0538f9a7a9e6499521650aa&acdnat=1341401895_4471445a987273c768f2d408869bd03d]--> Ryu KS, Ji L, Chang L, Ji TH. Molecular mechanism of LH/CG-receptor activation. Molecular and Cellular Endocrinology. 1996;125(1-2):93-100. | ||
| + | |||
| + | <div id="Petaja"></div> [11] <!--[http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1134190/pdf/biochemj00109-0219.pdf]--> Petäja-Repo UE, Merz WE, Rajaniemi HJ. Significance of the carbohydrate moiety of the rat ovarian luteinizing hormone/chorionic-gonadotropin receptor for ligand-binding specificity and signal transduction. The Biochemical Journal. 1993;292(3):839-844. | ||
| + | |||
| + | <div id="Dufau"></div> [12] Dufau ML. The hormone luteininizing hormone receptor. Annual review of Physiology. 1998;60(1):461-496. | ||
| + | |||
| + | <div id="Ladds"></div> [13] Ladds G, Goddard A, Davey J. Functional analysis of heterologous GPCR signaling pathways in yeast. Trends in Biotechnology. 2005;23(7):367-373. | ||
| + | |||
| + | <div id="Erlennach"></div> [14] Erlenbach I, Kostenis E, Schmidt C, Hamdan FF, Pausch MH, Wess J. Functional expression of M1, M3 and M5 muscarinic acetylcholine receptors in yeast. Journal of Neurochemistry. 2001;77(5):1327-1337. | ||
| + | |||
| + | <div id="RyoS"></div> [15] Ryo S, Ishii J, Iguchi Y, Fukuda N, Kondo A. Transplantation of the GAL regulon into G-protein signaling circuitry in yeast. Analytical Biochemistry. 2012;424(1):27-31. | ||
| - | <div id=" | + | <div id="Klis"></div> [16] Klis FM, Boorsma A, De Groot PWJ. Cell Wall Construction in Saccharomyces cerevisiae. Yeast. 2006;23(3):185-202. |
| - | <div id=" | + | <div id="Yurugi"></div> [17] Yurugi-Kobayashi T, Asada H, Shiroishi M, Shimamura T, Funamoto S, Katsuta N, et al. Comparison of functional non-glycosylated GPCRs expression in Pichia pastoris. Biochemical and Biophysical Research Communications. 2009;380(2):271-276. |
| - | <div id=" | + | <div id="Lanctot"></div> [18] Lanctot PM, Leclerc PC, Clement M, Auger-Messier M, Escher E, Leduc R, et al. Importance of N-glycosylation positioning for cell-surface expression, targeting, affinity and quality control of the human AT1 receptor. The Biochemical Journal. 2005;390(Pt 1):367-376. |
| - | <div id=" | + | <div id="Hamilton"></div> [19] Hamilton SR, Gerngross TU. Glycosylation engineering in yeast: the advent of fully humanized yeast. Current Opinion in Biotechnology. 2007;18(5):387-392. |
| - | <div id=" | + | <div id="Gilchrist"></div> [20] Gilchrist A, editor. GPCR molecular pharmacology and drug targeting : shifting paradigms and new directions. 5th ed. Hoboken, N.J: Wiley; 2010. |
| - | <div id=" | + | <div id="Feldmann"></div>[21]<!--[http://books.google.se/books?id=MuHG-_dkhLQC&pg=PA33&hl=sv&source=gbs_toc_r&cad=4#v=onepage&q&f=false]--> Feldmann H. Cellular Dynamics. In: Dr Koutsouki E, Dr Sendtko A, editors. Yeast molecular biology: a short compendium on basic features and novel aspects. Weinheim: Wiley-Balckwell; 2010. p. 33-82. |
| - | <div id=" | + | <div id="Bardwell"></div>[22]<!--[http://ac.els-cdn.com/S0196978104004486/1-s2.0-S0196978104004486-main.pdf?_tid=d8068d3f84f146effffac6cd6868c35d&acdnat=1341402231_d88ea9fd1dd1cda3d48888094307f3fc]--> Bardwell L. A walk-through of the yeast mating pheromone response pathway. Peptides.2004;25(9):1465-1476. |
| - | <div id="Han"></div>[http://ac.els-cdn.com/S0141022908000598/1-s2.0-S0141022908000598-main.pdf?_tid=4b2789324aefcec99a5983423600f171&acdnat=1341405624_045cdf6deefbf57c63f259595efa9d77] Han GH, Shin HJ, Kim SW. Optimization of bio-indigoproduction by recombinant E. coli harboring fmo gene. Enzyme and Microbial Technology. 2008;42(7):617-623. | + | <div id="Han"></div>[23]<!--[http://ac.els-cdn.com/S0141022908000598/1-s2.0-S0141022908000598-main.pdf?_tid=4b2789324aefcec99a5983423600f171&acdnat=1341405624_045cdf6deefbf57c63f259595efa9d77]--> Han GH, Shin HJ, Kim SW. Optimization of bio-indigoproduction by recombinant E. coli harboring fmo gene. Enzyme and Microbial Technology. 2008;42(7):617-623. |
| - | <div id="Ishii"></div>[http://www.springerlink.com.proxy.lib.chalmers.se/content/p4wyja04ny676nyt/fulltext.pdf] Berry A, Dodge TC, Pepsin M, Weyle W. Application of metabolic engineering to improve both the production and use of biotech indigo. Journal of Industrial Microbiology & Biotechnolog.2002;28(3):127-133. | + | <div id="Ishii"></div>[24]<!--[http://www.springerlink.com.proxy.lib.chalmers.se/content/p4wyja04ny676nyt/fulltext.pdf]--> Berry A, Dodge TC, Pepsin M, Weyle W. Application of metabolic engineering to improve both the production and use of biotech indigo. Journal of Industrial Microbiology & Biotechnolog.2002;28(3):127-133. |
{{Team:Chalmers-Gothenburg/footer}} | {{Team:Chalmers-Gothenburg/footer}} | ||
Latest revision as of 14:57, 7 August 2012
Contents |
Introduction
In this section we present some information needed to better understand the design of our biosensor. Firstly, a short introduction to G protein-coupled receptors will be given. Thereafter, the hCG hormone, its associated LH/CG receptor and their roles during pregnancy will be discussed. The construction of our hCG biosensor will require the assembly of several biological components in yeast, such as a functional human GPCR, a modified pheromone signaling pathway, and reporter genes encoding indigo-synthesizing enzymes. Hence, in this section, we will also present some background information and previous approaches within these areas.
G protein-coupled receptors
In our biosensor we will utilize the LH/CG receptor, which is a member of the family of G protein-coupled receptors (GPCRS). GPCRs are the largest class of cell-surface receptors and are used by all eukaryotic organisms, including yeast. They mediate responses to a wide variety of extracellular signals such as smell, taste, light, neurotransmitters and hormones [1] and they control important functions like heart rate and blood pressure. GPCRs are also the most common target for medicinal drugs today and nearly 30 % of all pharmaceuticals function by acting as modulators of GPCRs [2].
Structure
All GPCRs are made up of a single peptide with a cytosolic region, a ligand-binding extracellular region and seven transmembrane domains. The transmembrane domains are connected via three intracellular and three extracellular loops. Common for GPCRs is also that they mediate responses to extracellular signals by interacting and activating a trimeric GTP-binding protein (G protein). All G proteins have a similar structure and consist of three subunits called α, β and γ. These are in turn divided into several subclasses. For instance, there are more than 15 identified Gα subunits, which are sorted into four main classes: Gαi/o, Gαs, Gαq and Gα12 [3].
Signal transduction
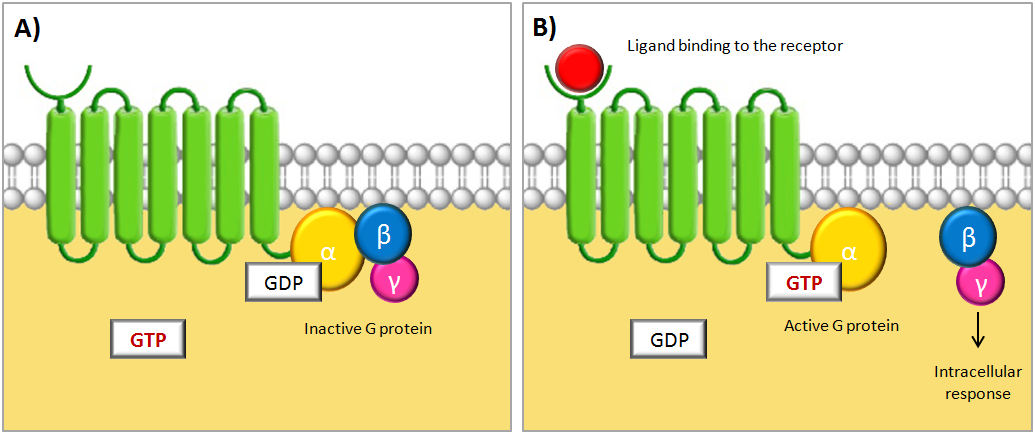
The activation of the G protein is achieved by a conformational change of the receptor after binding of a signal molecule to its ligand binding site. This change causes the α subunit to release its bound GDP and to exchange it for GTP. This in turn leads to a conformational change of the G protein itself, which is thereby activated. At this stage the subunits of the G protein can either dissociate or remain associated. In either case, the activated G protein then interacts with a target protein in the cell membrane, which relays the signal [1]. The figure below shows a schematic illustration of a G protein-coupled receptor and its interacting G protein.
Schematic illustrations of a G protein-coupled receptor (GPCR). The GPCR consists of a cytosolic region, a ligand-binding extracellular region and seven transmembrane domains. The receptor is associated with a trimeric GTP-binding protein (G protein) consisting of three subunits called α, β and γ. (A) When no signal molecule is present, the G protein binds to GDP and is inactive. (B) Upon binding of a signal protein to the ligand-binding site, a conformational change of the receptor occurs. This enables the receptor to interact with the α subunit of the associated G protein, which then exchanges its bound GDP to GTP. This activates the G protein and causes the βγ subunit to dissociate, thus enabling it to relay the signal by regulating the activity of additional intracellular signaling proteins. Figure is adapted from [1].
Human chorionic gonadotropin hormone


The hormone of interest when detecting pregnancy is human chorionic gonadotropin (hCG), which is a hormone that belongs to the family of glycoproteins [4]. During pregnancy, hCG will bind to the luteinizing hormone receptor (LH/CG receptor) that is localized on the yellow body and ensure the yellow body to maintains its progesterone production. This maintained level of progesterone stops menstrual bleeding and prepares the uterus lining for the implementation of the fertilized egg [5], [6].
All glycoprotein hormones share a common α subunit, but they also consist of a receptor-specifc β subunit. The hCG hormone is made up of an α and a β subunit that consist of 92 respectively 145 amino acid residues [8]. The term hCG actually refers to five independent molecules, all with various structure and biological function. The two dominating forms are regular hCG and hyperglycosylated hCG (H-hCG) but only regular hCG acts on the LH/CG receptor. Therefore our biosensor will only be able to detect regular hCG [8].
Luteinizing hormone receptor
The LH/CG receptor belongs to the family of G proteins-coupled receptors [9] and it is the receptor that we will utilize when constructing our biosensor. During pregnancy, the LH/CG receptor regulates levels of progesterone promoting growth of the fetus. The receptor is located in fetal organs and in fallopian and uterine tissues in women. It can also be found in regions of the adult brain such as hippocampus, hypothalamus and brain stem [6].
Structure
The extracellular domain is responsible for binding hCG and is characterized by a number of leucine-rich repeats. The transmembrane domains are needed for receptor activation and signal transduction after binding of the hormone. In addition, intracellular loop III is most likely physically linked to the corresponding G protein of the receptor [10]. The extracellular domain of the LH/CG receptor also contains six potential sites for glycosylation. Experiments have shown that the deglycoslyated LH/CG receptor binds native hCG with the same affinity as the glycosylated receptor and is able to activate the intracellular pathway. However, some of the glycosylation sites of the receptor may affect intracellular transport, membrane insertion or recycling of the receptor [11].
Activation and signal transduction
Researches indicates that hCG has an unique way to trigger its receptor. Initially, hCG binds to the extracellular domain of the LH/CG receptor which leads to an conformational change. This enables a secondary binding that activates the receptor and the signal is transduced through the transmembrane domains. This results in a conformational change of the intracellular loops and cytoplasmic C-terminal tail, which in turn activates the α subunit of a G protein, commonly Gαs or to varying degrees Gαq and Gαi [12]. Activation of Gα then results in the activation of the cAMP-dependent pathway [9].
Yeast as a model organism in the study of GPCRs
Saccharomyces cerevisiae (hereafter referred to as "yeast") is the most investigated eukaryotic organism and it is the host that we will utilize when expressing our human GPCR in this project. Mammalian and yeast GPCRs are structurally and functionally very similar and several heterologous GPCRs have successfully been expressed in yeast [2]. Because of this and due to its acceptability to genetic modifications, yeast is an attractive host for studying GPCRs [13]. In addition, the yeast pheromone response pathway, which is activated by endogenous yeast GPCRs, is very well-studied and has proven to bear a significant resemblance to mammalian GPCR signaling [14]. By exploiting the fact that several of the heterologously expressed GPCRs can be coupled with the pheromone pathway and by the use of different reporter genes, the yeast provides a simple tool for analysis of the GPCRs [15]. Here you can read more about the pheromone pathway.
Yeast-based expression of GPCRs does, however, have some limitations. Two of these, which we have identified as being of importance for this project, are discussed below.
The yeast cell wall
The cell wall of yeast can pose a problem in some situations when studying GPCRs. Apart from stabilizing the yeast cell, the cell wall also serves as a scaffold for external glycoproteins. These glycoproteins and especially their carbohydrate side-chains limit the permeability of the cell wall [16] and large molecules (Mr > 5000 Dalton) will not easily pass through it [13]. Hence, if a ligand of a heterologously expressed GPCR is larger than about 70-80 amino acid residues, there is risk that it will not be able to activate the receptor [13]. The hCG hormone has two subunits consisting of 92 and 145 amino acids respectively [8] and the size of the hormone could thus constitute a problem for the hCG biosensor. Removing the cell wall with enzymes could be a solution but it is impractical and often results in less viable yeast cells [13]. In our Methods section you can read about how we are planning to overcome this problem.
Glycosylation in yeast
Glycosylation is the most common post-translational modification of GPCRs [17] and approximately 95 % of all GPCRs are glycosylated [18]. Another possible limitation when using yeast to study GPCRs is that mammalian GPCRs expressed in yeast will be glycosylated in a significantly different way than when expressed in a mammalian cell [19]. There have also been cases of heterologous GPCRs not being glycosylated at all when expressed in yeast [20]. Thus, problems may arise when expressing heterologous GPCRs in yeast that require proper glycosylation for the correct ligand-binding and signal-transduction [20]. The LH/CG receptor, the GPCR used in this project, has a number of glycosylated sites and we have yet to see how and if the receptor will function without these when being expressed in yeast. However, according to Petäja-Repo et al. 1993 [11], glycosylation is required for neither the binding of the hCG hormone to the LH/CG receptor nor the correct signal transduction. On the other hand, its glycosylation might be important for the receptor to be correctly transported to and integrated in the cell membrane [11].
Yeast pheromone pathway
S.cerevisiae stably exists as either a haploid or a diploid. In both modes of existence, it reproduces asexually through a process called budding. This is an asymmetrical dividing process in which a mother cell gives rise to a smaller daughter cell [21]. Diploid yeast cells are stable but under stress conditions such as absence of nutrition, they can form haploid spores. In the haploid mode, yeast has two mating types, a and α (alpha) called MATa and MATα(alpha) respectively. The haploid yeast cells are capable to mate with the opposite mating type which results in the formation of a diploid zygote [22].
Mating between haploid cells is mediated by diffusible molecules called pheromones. The mating types secrete different pheromones (a- and α(alpha)-factors) and respond to the mating factor of the opposite mating type. The response occurs when the pheromones bind to a G protein-coupled receptor in the yeast cell membrane. The two mating types have different GPCRs (Ste2 and Ste3) each binding to the corresponding pheromone. The pheromones activate the GPCR which in turn creates an intracellular response. This intracellular signal transduction pathway in yeast is one the best understood signaling pathways in eukaryotes [2] and will also be utilized in the hCG biosensor. The whole pathway is illustrated in the Figure below.
Signal transduction
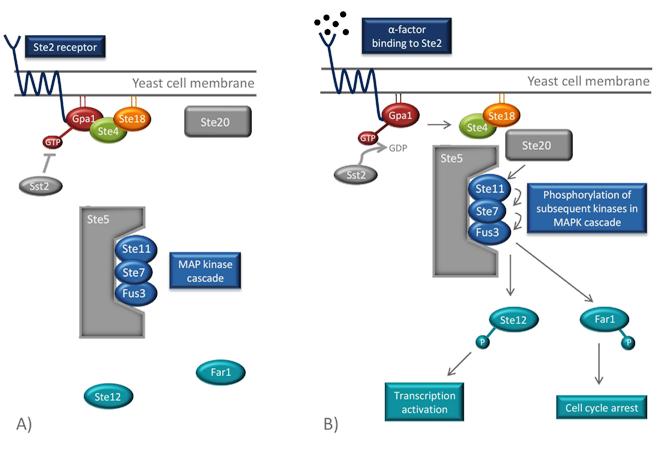
The GPCR of the MATa mating type is Ste2. Its associated G protein at the inside of the cell membrane consists of three subunits, Gpa1, Ste4 and Ste18, which correspond to mammalian Gα, Gβ and Gγ [2]. When α(alpha)-factors from the MATα(alpha) cell bind to the Ste2 receptor of the MATa-cell, a conformational change of the GPCR occurs. This activates the α(alpha) subunit of the G protein, the Gpa1, by exchanging its bound GDP for GTP. This leads to the dissociation of the Ste4-Ste18 complex, which in turn binds the effector proteins Ste5 and Ste20, through Ste4. Ste20 is a protein kinase and Ste5 is a scaffold protein that binds and organizes different kinases of the mitogen-activated protein kinase (MAPK) cascade. When the Ste4-Ste18 complex binds to both Ste5 and Ste20, the two proteins are moved towards each other. This enables Ste20 to phosphorylate the first kinase in the MAPK cascade, Ste11, which is bound to Ste5. Consequently, subsequent kinases (Ste7 and Fus3) of the cascade are phosphorylated, which ultimately results in the phosphorylation and activation Far1 and Ste12 [22].
Overview of the pheromone pathway in yeast. (A) In absence of pheromones, the G protein, consisting of Gpa1, Ste4 and Ste18, is inactive and can therefore not mobilize the components of the signaling pathway. (B) When a pheromone molecule binds to Ste2, the conformation of the receptor changes which leads to the exchange of GDP to GTP in Gpa1. This results in the dissociation of the Ste4-Ste18 complex. Ste4 can then bind to Ste20, a protein kinase, and to the scaffold protein Ste5, which organizes the kinases of the mitogen-activated protein kinase (MAPK) pathway. Ste20 phosphorylates the first kinase in the cascade (Ste11), which leads the phosphorylation of the subsequent kinases in the pathway (Ste7 and Fus3). Finally, Ste12, a transcription factor, is phosphorylated and transcription of pheromone-induced genes occurs. Another eject of the pathway is the phosphorylation of Far1 which results in cell cycle arrest. Sst2 is a negative feedback regulator and stimulates the hydrolysis of GTP in order to inactivate the signaling cascade. Figure is adapted from [2].
Indigo
Bio-indigo can be synthesized from the amino acid tryptophan in a few simple steps, which are outlined in the picture below. The first reaction is the conversion of tryptophan to indole, catalyzed by tryptophanase. The next part of the pathway is catalyzed by a mono- or dioxygenase and transforms indole to indoxyl. Indoxyl is then converted to indigo by several spontaneous reactions in the presence of oxygen [23], [24]. In order to produce bio-indigo in S. cerevisiae, genes encoding tryptophanase and a flavin-containing monooxygenase are inserted.
 "
"