Team:Tokyo Tech/Experiment/positivefeedback1
From 2012.igem.org
(→1.Construction) |
(→Co-culture assay) |
||
| (10 intermediate revisions not shown) | |||
| Line 4: | Line 4: | ||
<div class="whitebox"> | <div class="whitebox"> | ||
| - | = | + | <div class="whitebox"> |
| - | + | =Co-culture assay= | |
| + | <div id="tokyotech" style=" font:Arial ;left ; font-size: 15px; color: #000000; padding: 30px;"> | ||
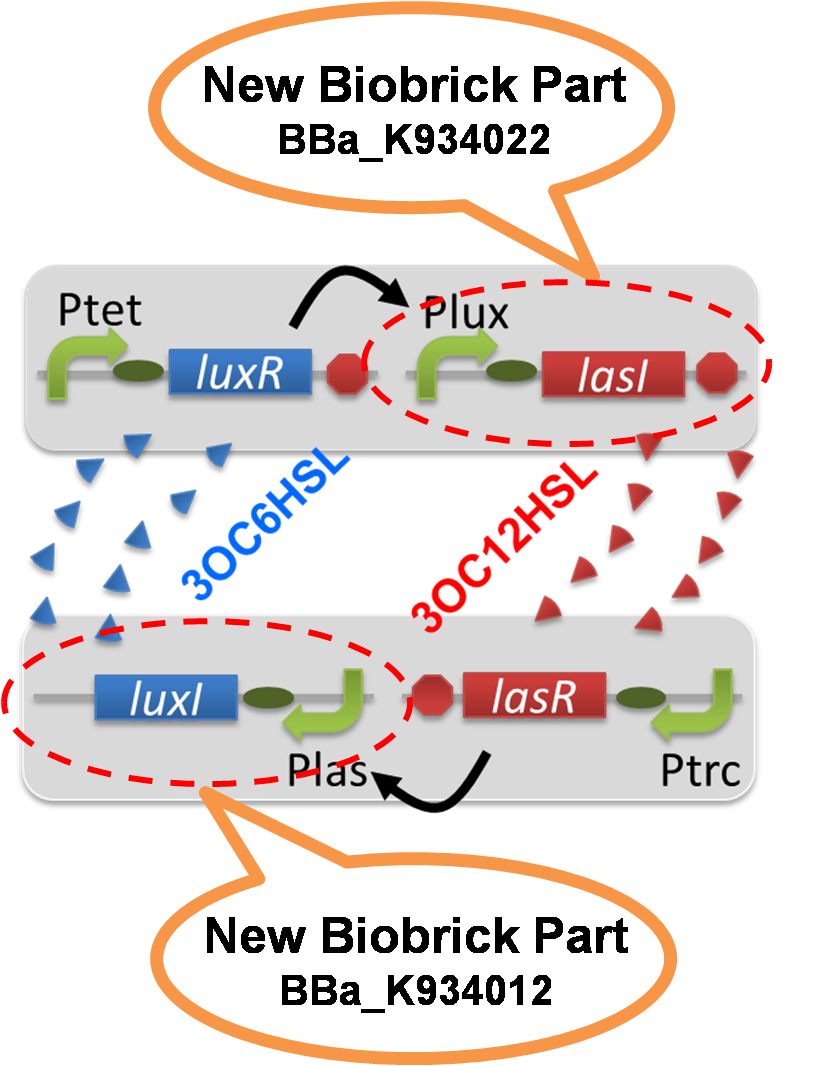
| - | To accomplish complete positive feedback system, we mixed and co-cultured Plux-LasI cell and Plas-LuxI cell (Fig2-1-3-1-1). As a control co-culture, ⊿P-LasI cell and ⊿P-LuxI cell were mixed. For a trigger of the positive feedback system, we added the initial | + | [[File:positivefeedbackassay17tokyotech.png|200px|thumb|right|Fig2-1-3-1-1, Positive feedback system in the cell-cell communication]] |
| - | + | <br><br> | |
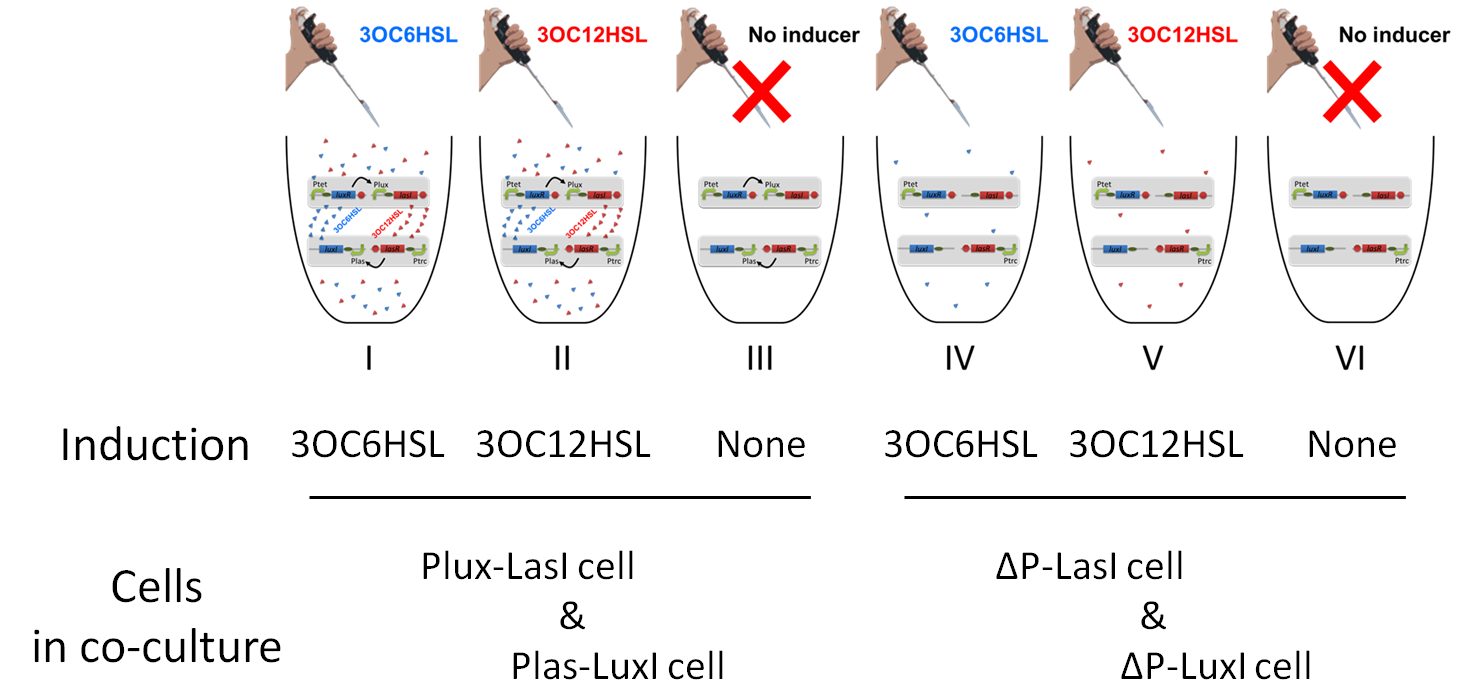
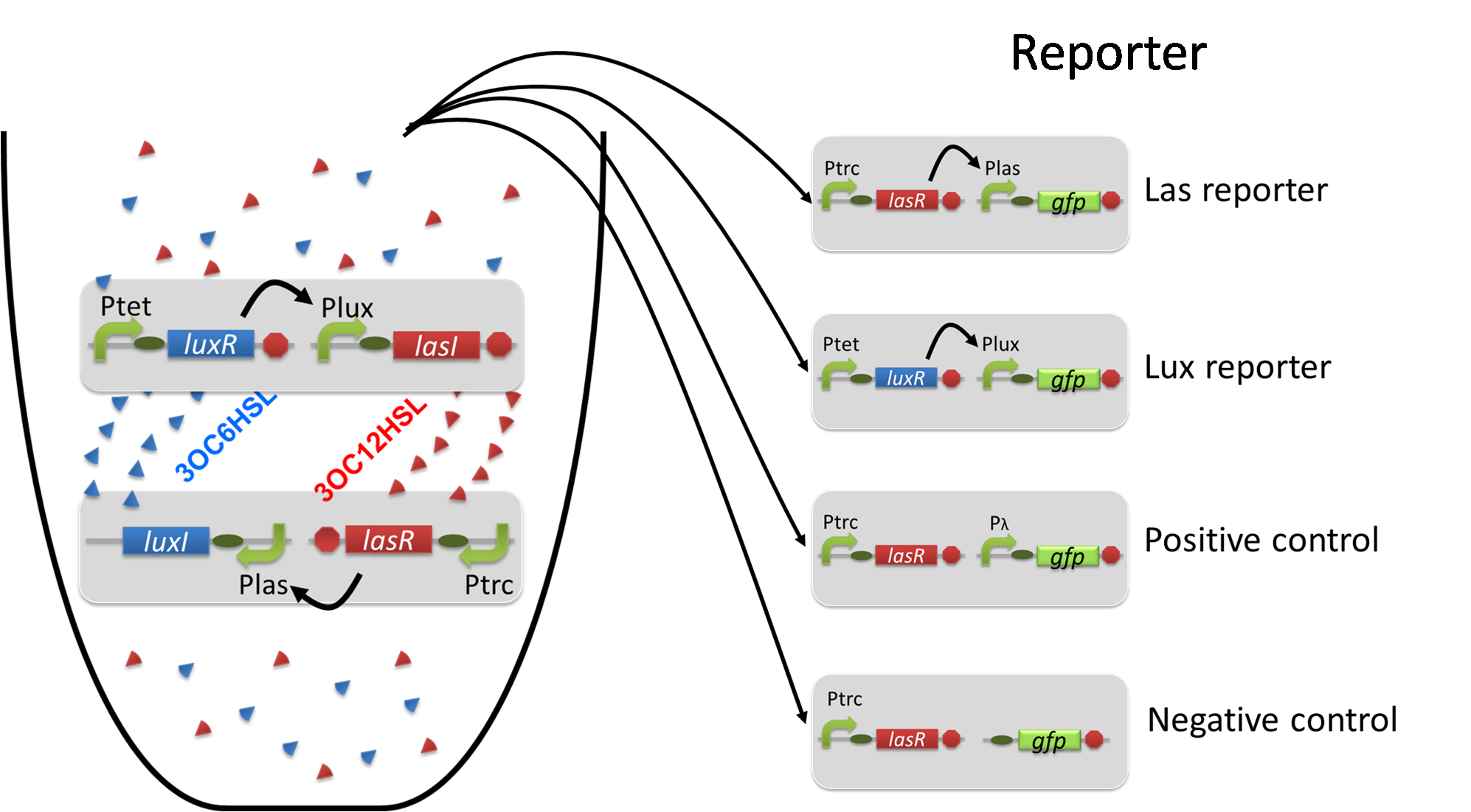
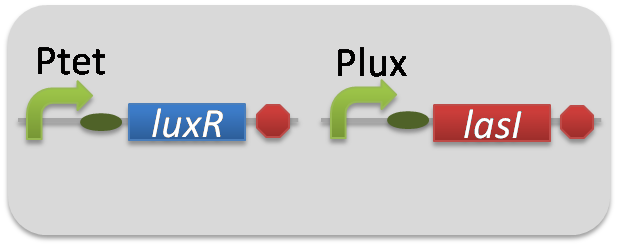
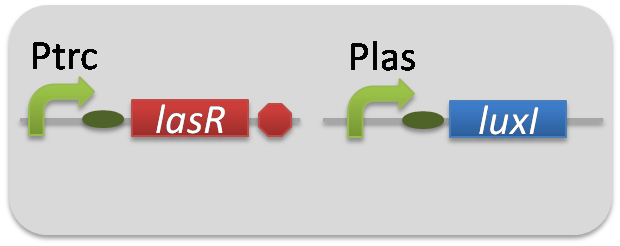
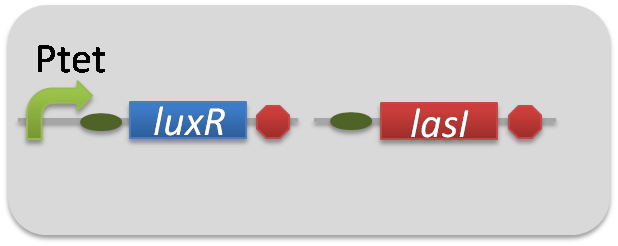
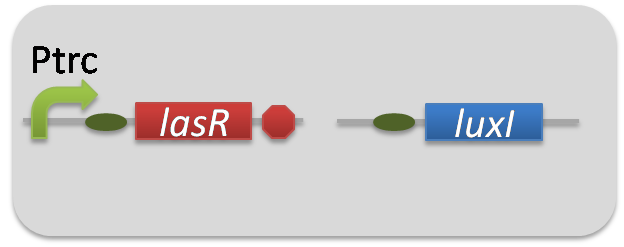
| + | To accomplish complete positive feedback system, we mixed and co-cultured Plux-LasI cell and Plas-LuxI cell (Fig2-1-3-1-1). As a control co-culture, ⊿P-LasI cell and ⊿P-LuxI cell were mixed. For a trigger of the positive feedback system, we added the initial dose of 3OC6HSL (5nM) or 3OC12HSL (2.5nM) to the co-cultures. Including no-induction controls, we thus prepared six conditions (Fig2-1-3-1-8). To confirm that both 3OC6HSL and 3OC12HSL are produced in the positive feedback, the signals content in the supernatants of the co-cultures were evaluated by Las reporter strain and Lux reporter strain (Fig2-1-3-1-9). | ||
| - | [[File:positivefeedbackassay27tokyotech.png| | + | [[File:positivefeedbackassay27tokyotech.png|700px|thumb|center|Fig2-1-3-1-8, Six conditions prepared for positive feedback assay |
]] | ]] | ||
| - | [[File:positivefeedbackassay28tokyotech.png|500px|thumb| | + | [[File:positivefeedbackassay28tokyotech.png|500px|thumb|center|Fig2-1-3-1-9, How to evaluate the positive feedback |
]] | ]] | ||
In these co-cultures, following behavior would be expected. In the condition I, 3OC6HSL induces 3OC12HSL production of Plux-LasI cell. Then, 3OC12HSL synthesized by Plux-LasI cell induces Plas-LuxI cell. The concentration of 3OC6HSL thus increases compared to the initial amount. Increased amount of 3OC6HSL induces higher production of 3OC12HSL. As a result, the production of both signals increased in the condition I. In this situation, the concentration of 3OC6HSL is higher than the initial concentration of 3OC6HSL (ideal positive feedback behavior). Similarly in the condition II, total amount of 3OC12HSL also increases compared to the initial amount. In the conditions III and VI, no signal is produced because of the lack of inducer. In conditions IV and V, little amount of 3OC6HSL and 3OC12HSL are remained without degradation, respectively. | In these co-cultures, following behavior would be expected. In the condition I, 3OC6HSL induces 3OC12HSL production of Plux-LasI cell. Then, 3OC12HSL synthesized by Plux-LasI cell induces Plas-LuxI cell. The concentration of 3OC6HSL thus increases compared to the initial amount. Increased amount of 3OC6HSL induces higher production of 3OC12HSL. As a result, the production of both signals increased in the condition I. In this situation, the concentration of 3OC6HSL is higher than the initial concentration of 3OC6HSL (ideal positive feedback behavior). Similarly in the condition II, total amount of 3OC12HSL also increases compared to the initial amount. In the conditions III and VI, no signal is produced because of the lack of inducer. In conditions IV and V, little amount of 3OC6HSL and 3OC12HSL are remained without degradation, respectively. | ||
| - | + | <br><br><br> | |
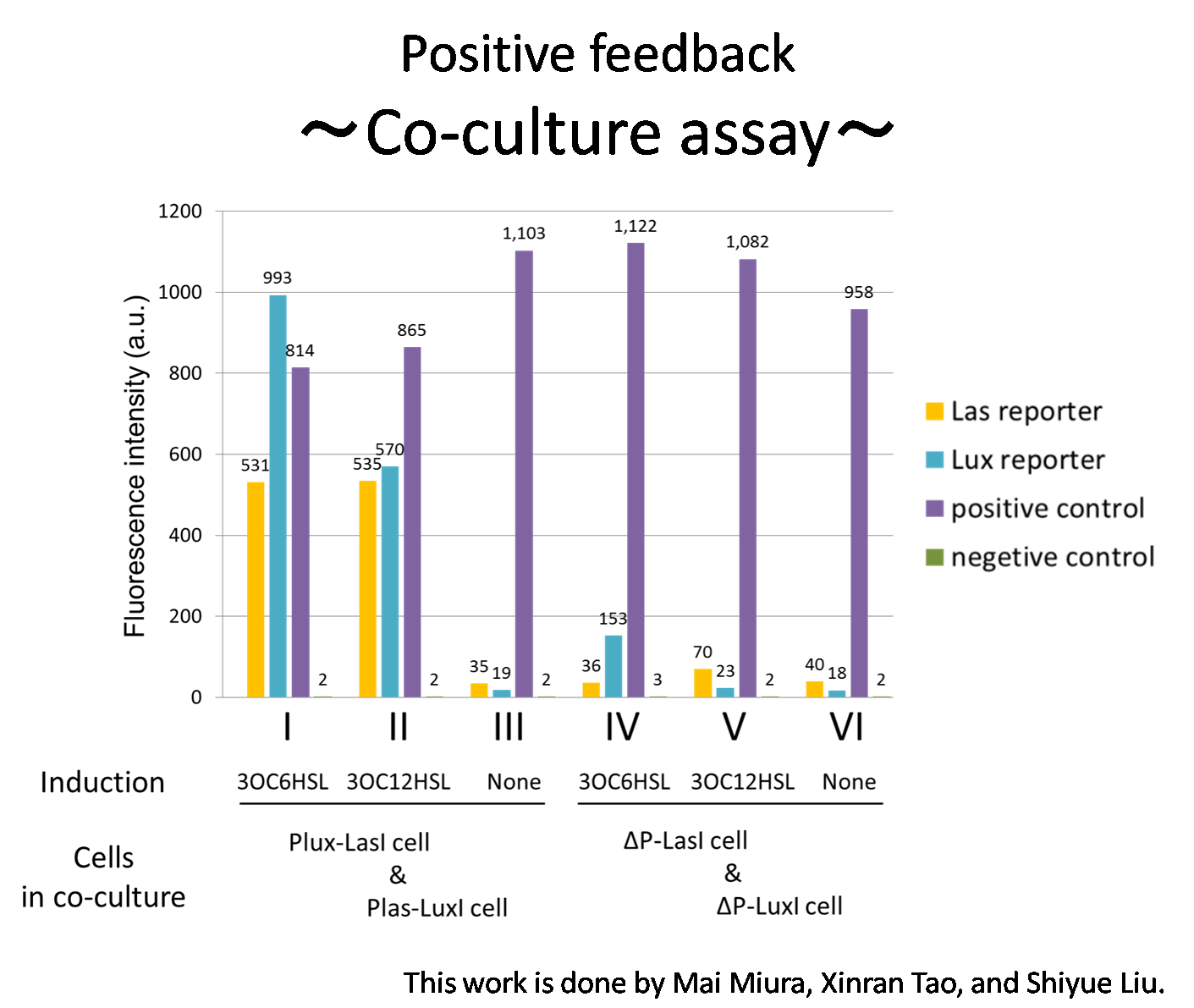
[[File:positivefeedbackassay31tokyotech.png|550px|thumb|right|Fig2-1-3-1-10, Positive feedback assay | [[File:positivefeedbackassay31tokyotech.png|550px|thumb|right|Fig2-1-3-1-10, Positive feedback assay | ||
]] | ]] | ||
| - | + | <br> | |
Fig2-1-3-1-10 shows that the fluorescence intensity of the Lux reporter cell in the condition I was higher than that in the condition IV. Similarly, the fluorescence intensity of the Las reporter in the condition II was higher than that in the condition V. | Fig2-1-3-1-10 shows that the fluorescence intensity of the Lux reporter cell in the condition I was higher than that in the condition IV. Similarly, the fluorescence intensity of the Las reporter in the condition II was higher than that in the condition V. | ||
From these results and circuit design described above, it is suggested that the appearance of positive feedback where cooperation of Plas-LuxI cell and Plux-LasI cell increase the production of 3OC6HSL and 3OC12HSL. | From these results and circuit design described above, it is suggested that the appearance of positive feedback where cooperation of Plas-LuxI cell and Plux-LasI cell increase the production of 3OC6HSL and 3OC12HSL. | ||
<br><br><br><br><br> | <br><br><br><br><br> | ||
<br><br><br><br><br><br><br><br><br><br><br><br> | <br><br><br><br><br><br><br><br><br><br><br><br> | ||
| - | |||
=Materials & Methods= | =Materials & Methods= | ||
| - | [[https://2012.igem.org/Team:Tokyo_Tech/Project# | + | [[https://2012.igem.org/Team:Tokyo_Tech/Project#Positive_feedback_assay_.7ECo-culture_assay.7E Go to the project page "Co-culture assay"]] |
==1.Construction== | ==1.Construction== | ||
| Line 79: | Line 80: | ||
=Protocol= | =Protocol= | ||
| - | [[https://2012.igem.org/Team:Tokyo_Tech/Project# | + | [[https://2012.igem.org/Team:Tokyo_Tech/Project#Positive_feedback_assay_.7ECo-culture_assay.7E Go to the project page "Co-culture assay"]] |
1. collect liquid culture | 1. collect liquid culture | ||
| - | <div id="tokyotechprotocol" style=" font:bold ;left ; font-size: | + | <div id="tokyotechprotocol" style=" font:bold ;left ; font-size: 15px; color: #000000; padding: 10px;"> |
1.1 Prepare overnight culture of inducer cells at 37°C for 12hours. | 1.1 Prepare overnight culture of inducer cells at 37°C for 12hours. | ||
| Line 101: | Line 102: | ||
</div> | </div> | ||
2 Reporter assay | 2 Reporter assay | ||
| - | <div id="tokyotechprotocol" style=" font:bold ;left ; font-size: | + | <div id="tokyotechprotocol" style=" font:bold ;left ; font-size: 15px; color: #000000; padding: 10px;"> |
2.1 Prepare overnight culture of reporter cells at 37°C for 12hours. | 2.1 Prepare overnight culture of reporter cells at 37°C for 12hours. | ||
| Line 118: | Line 119: | ||
</div> | </div> | ||
| - | [[https://2012.igem.org/Team:Tokyo_Tech/Project# | + | [[https://2012.igem.org/Team:Tokyo_Tech/Project#Positive_feedback_assay_.7ECo-culture_assay.7E Go to the project page "Co-culture assay"]] |
Latest revision as of 01:43, 27 October 2012
Contents |
Co-culture assay
To accomplish complete positive feedback system, we mixed and co-cultured Plux-LasI cell and Plas-LuxI cell (Fig2-1-3-1-1). As a control co-culture, ⊿P-LasI cell and ⊿P-LuxI cell were mixed. For a trigger of the positive feedback system, we added the initial dose of 3OC6HSL (5nM) or 3OC12HSL (2.5nM) to the co-cultures. Including no-induction controls, we thus prepared six conditions (Fig2-1-3-1-8). To confirm that both 3OC6HSL and 3OC12HSL are produced in the positive feedback, the signals content in the supernatants of the co-cultures were evaluated by Las reporter strain and Lux reporter strain (Fig2-1-3-1-9).
In these co-cultures, following behavior would be expected. In the condition I, 3OC6HSL induces 3OC12HSL production of Plux-LasI cell. Then, 3OC12HSL synthesized by Plux-LasI cell induces Plas-LuxI cell. The concentration of 3OC6HSL thus increases compared to the initial amount. Increased amount of 3OC6HSL induces higher production of 3OC12HSL. As a result, the production of both signals increased in the condition I. In this situation, the concentration of 3OC6HSL is higher than the initial concentration of 3OC6HSL (ideal positive feedback behavior). Similarly in the condition II, total amount of 3OC12HSL also increases compared to the initial amount. In the conditions III and VI, no signal is produced because of the lack of inducer. In conditions IV and V, little amount of 3OC6HSL and 3OC12HSL are remained without degradation, respectively.
Fig2-1-3-1-10 shows that the fluorescence intensity of the Lux reporter cell in the condition I was higher than that in the condition IV. Similarly, the fluorescence intensity of the Las reporter in the condition II was higher than that in the condition V.
From these results and circuit design described above, it is suggested that the appearance of positive feedback where cooperation of Plas-LuxI cell and Plux-LasI cell increase the production of 3OC6HSL and 3OC12HSL.
Materials & Methods
[Go to the project page "Co-culture assay"]
1.Construction
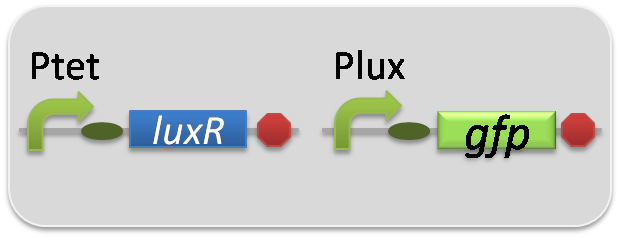
pSB6A1-Ptet-LuxR / pSB3K3-Plux-LasI (JM2.300)…Plux-LasI cell
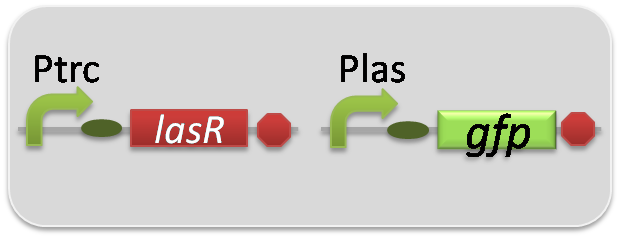
pSB6A1-Ptrc-LasR / pSB3K3-Plas-LuxI (JM2.300)…Plas-LuxI cell
pSB6A1-Ptet-LuxR / pSB3K3-ΔP-LasI (JM2.300)…ΔP-LasI cell
pSB6A1-Ptrc-LasR / pSB3K3-ΔP-LuxI (JM2.300)…ΔP-LuxI cell
pSB6A1-Ptrc-LasR / pSB3K3-Plas-GFP (JM2.300)…Las reporter cell
pSB6A1-Ptet-LuxR / pSB3K3-Plux-GFP (JM2.300)…Lux reporter cell
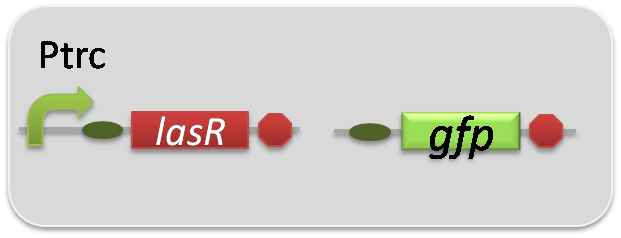
pSB6A1-Ptrc-LasR / pSB3K3-ΔP-GFP (JM2.300)…negative control
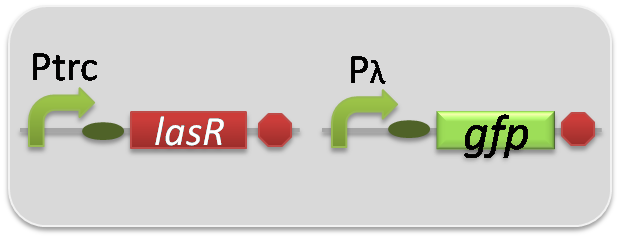
pSB6A1-Ptrc-LasR / pSB3K3-pλ-GFP (JM2.300)…positive control
2.Strain
JM2.300
Protocol
[Go to the project page "Co-culture assay"]
1. collect liquid culture
1.1 Prepare overnight culture of inducer cells at 37°C for 12hours.
1.2 Take 30μl of the overnight culture of inducer cells into LB (3ml) + antibiotics (Amp 50μg/ml + Kan 30μg/ml). (→fresh culture)
1.3 Incubate the flesh culture of inducer cells until the observed OD600 reaches around 0.50. Centrifuge the cells at 5000g, 25°C, 1 min, suspend it with 1ml LB + antibiotics (Amp 50μg/ml + Kan 30μg/ml).
1.4 According to the Fig.3-1-3-1-1, take 30μl cell suspensions into LB(3ml) + antibiotics (Amp 50μg/ml + Kan 30μg/ml) + 3OC6HSL (5nM) or OC6HSL(2.5nM)
1.5 Incubate the inducer cell for another 4 hours at 37°C.
1.6 Centrifuge the inducer cell at 9000g, 4°C, 1 min, and filter the cultured cell.
1.7 Dilute the filtrate by LB + antibiotics (Amp 50μg/ml + Kan 30μg/ml) in 1:30.
2 Reporter assay
2.1 Prepare overnight culture of reporter cells at 37°C for 12hours.
2.2 Take 30μl of the overnight culture of reporter cells into LB (3ml) + antibiotics (Amp 50μg/ml + Kan 30μg/ml). (→fresh culture)
2.3 Incubate the flesh culture of reporter cells until the observed OD600 reaches around 0.50, and gather the supernatant of culture of inducer cells.
2.4 Centrifuge the reporter cells at 5000g, 25°C, 1 min, and take it into LB + antibiotics (Amp 50μg/ml + Kan 30μg/ml).
2.5 Add 30μl samples of process 2.4 to filtrate + LB (3ml) + antibiotics (Amp 50μg/ml + Kan 30μg/ml) from process 1.7
2.6 Incubate the reporter cells for 4 hours at 37°C.
2.7 Flow cytometer measurements for GFP expression of reporter cells.
 "
"