Team:Peking/Project/Communication/Results
From 2012.igem.org
m |
|||
| Line 2: | Line 2: | ||
<script type="text/javascript"> | <script type="text/javascript"> | ||
| - | sublists_Now = | + | sublists_Now = 2; |
var subsubitem=subfirst.getElementsByTagName('ul')[sublists_Now].getElementsByTagName('a')[2]; | var subsubitem=subfirst.getElementsByTagName('ul')[sublists_Now].getElementsByTagName('a')[2]; | ||
subsubitem.style.color='#60b0f0'; | subsubitem.style.color='#60b0f0'; | ||
Latest revision as of 05:12, 26 October 2012
Response to Bio-luminescence:
A Quick Proof of Concept
As shown in Figure 1, GFP expression was repressed by the Luminesensor expressed in light sensing cells under either bio-luminescence (1) or dim LED (3) (as the positive control), indicating that the light-sensing cell can sense bio-luminescence quite well. The cells in our set-up with no light emitting cells (2), or just inoculated test tubes wrapped with aluminum foil (4) have expressed GFP, indicating that our device wrapped with aluminum foil effectively excludes the influence of other sources of light.
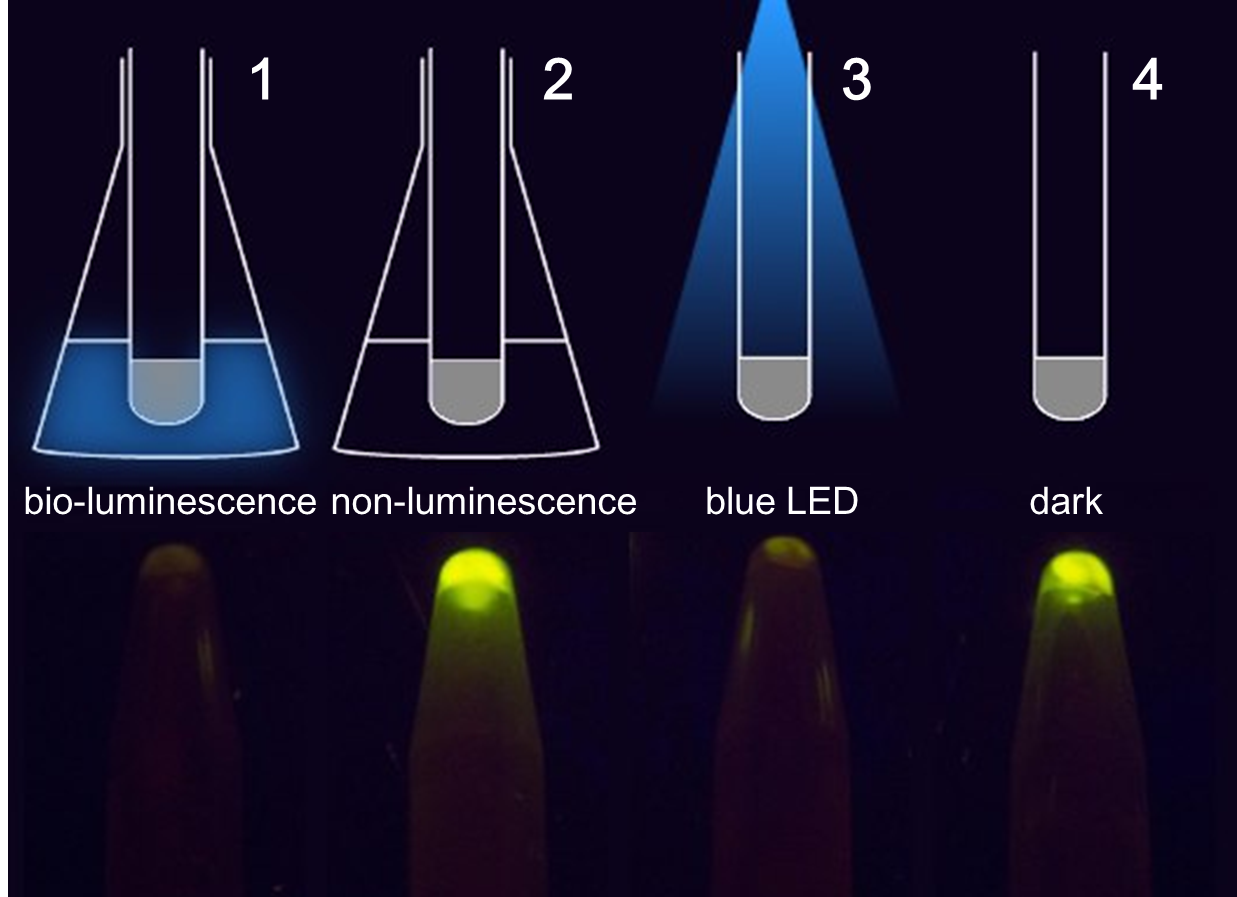
Figure 1. The treatment to each group(upper) and the result(lower). 1 and 2 contain cells grown in the communication set-up, but for group 2 there is no light emitting cell in the conical flask but only wild-type DH5α. 3 and 4 contain the same light sensing cells grown in test tubes under dim LED (3) or in pure darkness (4).
To obtain more quantitative data, we measured the GFP expression level using a Tecan infinite 200 reader. As is shown in the graph below (Figure 2), the expression level of GFP in darkness (in our device with no glowing cells) is about 200-fold higher than that of the cells under bio-luminescence.
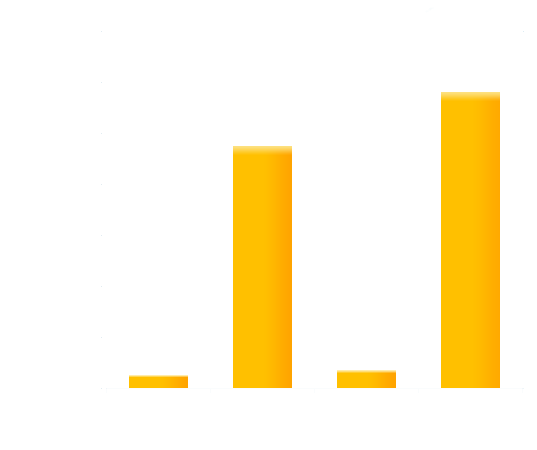
Figure 2. Quantitative measurement of repression effects of bio-luminescence on the expression of GFP in receiver cells.
Detailed characterization
Since we had confirmed that our sensing cell (cells expressing the Luminesensor and the reporter gene) could respond to bio-luminescence quite well, we were interested in more detailed information regarding our light communication system.
1. Spectrum
Firstly, although the spectrum of the luminescence produced by lux genes was given in scientific literature, there may be some slight variation when the genes are expressed in E.coli grown in LB medium. Cambridge 2010 iGEM team did not provide the spectrum for their luxbrick, therefore, in order for us to confirm that the luminescence from our light emitting cell is able to meet the required wavelength. Also, as a supplementary characterization for Cambridge 2010 iGEM team’s luxbrick part, we measured the spectrum of the luminescence emitted by glowing cell.
In the spectrum diagram (Figure 3), we can see an emission peak at near 485nm, which matches the maximum absorption wavelength of our Luminesensor.
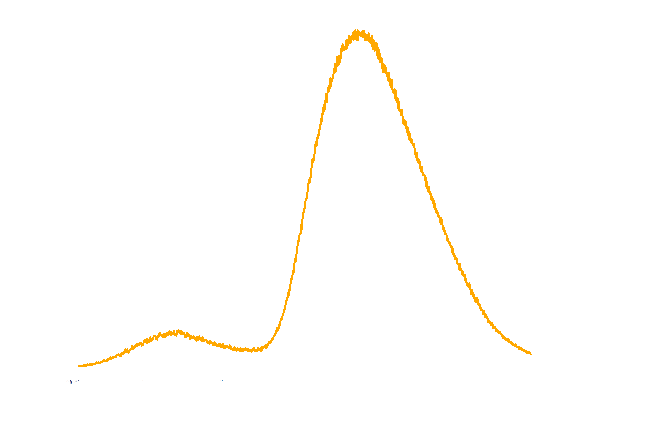
Figure 3. TOP10 cells harboring the luxbrick were cultured in LB medium and induced with L-arabinose at 10-3M. 10 hours after induction, the glowing cells were measured for spectrum using SHIMADZU RF5301PC Spectrofluorophotometer.
2. Time Courses
Then we wanted to figure out when our light emitting cell induced with L-arabinose would begin to glow. Although 2010 Cambridge iGEM team has provided a graph showing the evolution of luminescence intensity with time during the light emitting process, we wanted to create a more visualized characterization of the luxbrick. Therefore, we make a short movie in order to show the time course of our light emitting cell transformed with luxbrick.
As we can see in the movie, the cells begin to glow 9 hours after induction, and the entire visible glowing process lasts about 10 hours. So in the light communication experiment, we decided to put light sensing cell amid the light emitting cell 9 hours after induction in order to maintain illumination on the light sensing cell, especially in their logarithmic phase.
if you can not view this video,please click HERE.
Movie1. the time course of our light emitting cell
Then we go on to the time course of light sensing cells’ behavior.
In the short video below, the reaction of our light sensing cell is shown on the right of each group. And the graph below the photos shows the light intensity (blue graph) and the GFP level (green graph) as a function of time.
if you can not view this video,please click HERE.
Movie 2. The time course of the whole system (Newly inoculated light sensing cells culture was immersed into the light emitting cells when the latter began to glow. Every 20 minutes, 0.5ml light sensing cells culture were taken out from our device and harvested in the bottom of an EP tube. Then GFP expression was observed under blue light excitation.)
As shown in the video, 9 hours later the cells in the flask began to glow, and light sensing cells were then inoculated in the device. 7 hours after that, clear cell pellets could be observed at the bottom of EP tubes (red circle), indicating that the light sensing cell reached a high quantity.
The experiment group (left) with a luminescence pulse lasting 8 hours from light emitting cell, as expected, didn’t express GFP, while the control group (right) in the same device with nonluminous cell, began to express GFP 8 hours after inoculation.
3. Response threshold and regulation through light intensity
As described before, the response threshold value of our Luminesensor was not found simply using blue LED and attenuating filters, because the Luminesensor is so sensitive to dim light, which cannot be detected by the photometer we used.
It is difficult to control the intensity of very dim light simply using attenuating filters, so we managed to find the threshold and therefore regulate the response intensity using bio-luminescence as light source based on our light-communication system.
Light emitting cell broth was diluted to create different light intensity, represented by the dilution ratio. (e.g. 0.001 indicates the weakest light intensity) With this method we managed to get closer to the linear area of our sensor’s response. We succeeded in regulating the gene expression level by changing the light intensity.
The photo below shows the GFP level to the dilution ratio of light emitting cell

Figure 4. Serial dilution of light emitting cell gradually inhibited the expression of GFP in receiver cells.
Here are quantitative data of effects of serial dilution of light emitting cell on the expression of GFP in receiver cells
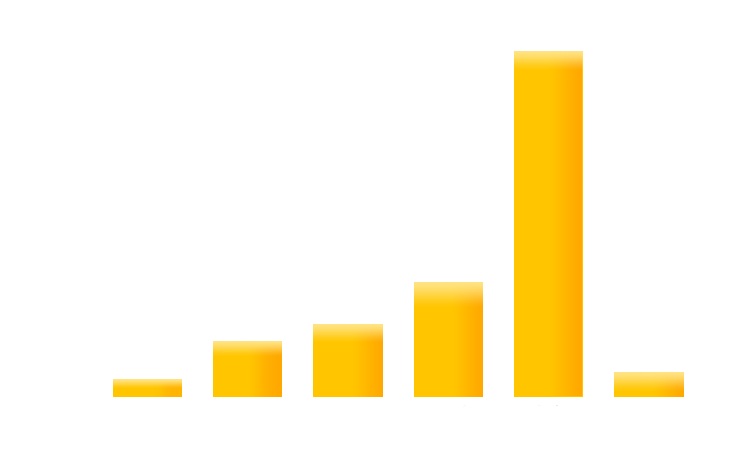
Figure 5. Measurement of relative GFP level to the dilution ratio of light emitting cell using a Tecan infinite 200 reader
Conclusion
So far we have confirmed and characterized light communication between cells through many different ways and all the results show that we indeed realized cell-cell communication through light.
 "
"














