Team:Minnesota/Project/UV Absorption
From 2012.igem.org
(Difference between revisions)
m |
|||
| (16 intermediate revisions not shown) | |||
| Line 132: | Line 132: | ||
<div id="MainBoxContent" style="background-color:white;"> | <div id="MainBoxContent" style="background-color:white;"> | ||
| - | <div style="position:absolute;top:0px;margin-left:50px; width:600px; height:450px; overflow-y:scroll; text-align:left;"> <!---START HERE---> | + | <div style="position:absolute;top:0px;margin-left:50px; width:600px; height:450px; overflow-y:scroll; text-align:left;overflow-x:hidden;"> <!---START HERE---> |
<h1>Synthesizing UV-Protective Compounds in Bacteria</h1><br> | <h1>Synthesizing UV-Protective Compounds in Bacteria</h1><br> | ||
| Line 144: | Line 144: | ||
Our research has focused on two novel biosynthetic pathways found in two distinct algal species. A pathway ending in the production of two UV-protective compounds, shinorine and mycosporine-glycine, was cloned from <i>Anabaena varibalis</i>. A second pathway leading to the production of the unrelated UV-screening compound scytonemin was cloned from <i>Nostoc punctiforme</i>. Our objective is to develop novel and effective production platforms for these compounds, some of which are currently used in expensive sunscreens and lotions. These compounds are used both for the UV-absorptive properties as well as their role as potent antioxidants.</p> | Our research has focused on two novel biosynthetic pathways found in two distinct algal species. A pathway ending in the production of two UV-protective compounds, shinorine and mycosporine-glycine, was cloned from <i>Anabaena varibalis</i>. A second pathway leading to the production of the unrelated UV-screening compound scytonemin was cloned from <i>Nostoc punctiforme</i>. Our objective is to develop novel and effective production platforms for these compounds, some of which are currently used in expensive sunscreens and lotions. These compounds are used both for the UV-absorptive properties as well as their role as potent antioxidants.</p> | ||
| - | + | <img src="http://i1158.photobucket.com/albums/p607/iGEM_MN/1Bac.png"> | |
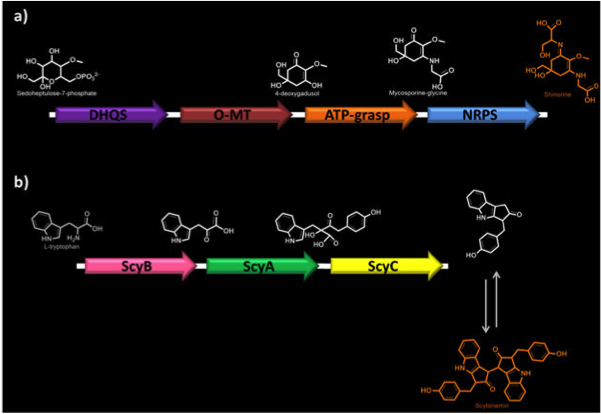
<p><b>Figure 1. Biosynthetic pathways of MAAs.</b> a) Shinorine biosynthesis. DHQS, dehydroquinate synthase; O-MT, O-methyltransferase; ATP-grasp; NRPS, non-ribosomal peptide synthase. b) Scytonemin biosynthesis. ScyA (NpR1276), acetolactate synthetase; ScyB (NpR1275), leucine dehydrogenase; ScyC (NpR1274), protein of unknown function.</p> | <p><b>Figure 1. Biosynthetic pathways of MAAs.</b> a) Shinorine biosynthesis. DHQS, dehydroquinate synthase; O-MT, O-methyltransferase; ATP-grasp; NRPS, non-ribosomal peptide synthase. b) Scytonemin biosynthesis. ScyA (NpR1276), acetolactate synthetase; ScyB (NpR1275), leucine dehydrogenase; ScyC (NpR1274), protein of unknown function.</p> | ||
| Line 156: | Line 156: | ||
<p><b>Parts List</b><br> | <p><b>Parts List</b><br> | ||
| - | BBa_K814000     dehydroquinate synthase (DHQS) generator <br> | + | BBa_K814000 dehydroquinate synthase (DHQS) generator <br> |
| - | BBa_K814001     ATP-grasp (ATPG) generator <br> | + | BBa_K814001 ATP-grasp (ATPG) generator <br> |
| - | BBa_K814002     dehydroquinate O-methyltrasferase (O-MT) generator <br> | + | BBa_K814002 dehydroquinate O-methyltrasferase (O-MT) generator <br> |
| - | BBa_K814003     shinorine non-ribosomal peptide synthase (NRPS) generator <br> | + | BBa_K814003 shinorine non-ribosomal peptide synthase (NRPS) generator <br> |
| - | BBa_K814004     mycosporine-glycine biosynthetic pathway <br> | + | BBa_K814004 mycosporine-glycine biosynthetic pathway <br> |
| - | BBa_K814005     shinorine biosynthetic pathway <br> | + | BBa_K814005 shinorine biosynthetic pathway <br> |
| - | BBa_K814006     negative control for mycosporine-like amino acid biosynthesis <br> | + | BBa_K814006 negative control for mycosporine-like amino acid biosynthesis <br> |
| - | BBa_K814007     ScyA (acetolactate synthase) generator <br> | + | BBa_K814007 ScyA (acetolactate synthase) generator <br> |
| - | BBa_K814008     ScyB (leucine dehydrogenase) generator <br> | + | BBa_K814008 ScyB (leucine dehydrogenase) generator <br> |
| - | BBa_K814009     ScyC generator <br> | + | BBa_K814009 ScyC generator <br> |
| - | BBa_K814010     partial scytonemin biosynthetic pathway, scyCB <br> | + | BBa_K814010 partial scytonemin biosynthetic pathway, scyCB <br> |
| - | BBa_K814011     scytonemin biosynthetic pathway, scyBAC <br></p> | + | BBa_K814011 scytonemin biosynthetic pathway, scyBAC <br></p> |
<p><b>Results</b><br> | <p><b>Results</b><br> | ||
| - | + | <img src="http://i1158.photobucket.com/albums/p607/iGEM_MN/2_Bac.png"> | |
<p><b>Figure 2. HPLC analysis of E. coli expressing MAA biosynthetic genes.</b> Cell extracts of E. coli transformed with +DOA (DHQS, O-methyl transferase, and ATP-grasp) and +DOAN (DOA, and NRPS) showed production and 4-deoxygadusol (a) and mycosporine-glycine (b). Shinorine was not detected in the +DOAN expressing cells. Samples were compared against a +DA negative control (this construct lacks the ability to produce the 4-deoxygadusol intermediate). Cells expressing +ABC (ScyA, ScyB, ScyC) did not show scytonemin production. Data was compared with previous results from Balskus and Walsh, as a standard could not be obtained. </p> | <p><b>Figure 2. HPLC analysis of E. coli expressing MAA biosynthetic genes.</b> Cell extracts of E. coli transformed with +DOA (DHQS, O-methyl transferase, and ATP-grasp) and +DOAN (DOA, and NRPS) showed production and 4-deoxygadusol (a) and mycosporine-glycine (b). Shinorine was not detected in the +DOAN expressing cells. Samples were compared against a +DA negative control (this construct lacks the ability to produce the 4-deoxygadusol intermediate). Cells expressing +ABC (ScyA, ScyB, ScyC) did not show scytonemin production. Data was compared with previous results from Balskus and Walsh, as a standard could not be obtained. </p> | ||
| - | + | <img src="http://i1158.photobucket.com/albums/p607/iGEM_MN/3Bac.png"> | |
<p><b>Figure 3. UV sensitivity screens of E. coli cultures producing MAAs.</b> Preliminary results for the secondary screen showed increased survival in cells producing mycosporine-glycine when compared with +DO and +DA negative controls. Cells expressing +ABC did not show increased survivability.</p> | <p><b>Figure 3. UV sensitivity screens of E. coli cultures producing MAAs.</b> Preliminary results for the secondary screen showed increased survival in cells producing mycosporine-glycine when compared with +DO and +DA negative controls. Cells expressing +ABC did not show increased survivability.</p> | ||
| - | + | <img src="http://i1158.photobucket.com/albums/p607/iGEM_MN/cellimages.jpg"> | |
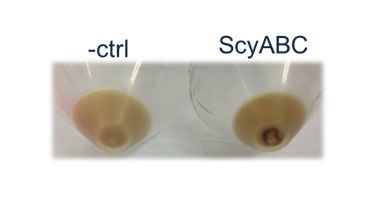
<p><b>Figure 4. Visual inspection of E. coli cells containing the scytonemin pathway.</b> Although HPLC and growth tests were negative, cells expressing +ABC were seen to be expressing a deep brown product that could easily be visualized (below). The control is a cell culture expressing a +DA plasmid vector. </p> | <p><b>Figure 4. Visual inspection of E. coli cells containing the scytonemin pathway.</b> Although HPLC and growth tests were negative, cells expressing +ABC were seen to be expressing a deep brown product that could easily be visualized (below). The control is a cell culture expressing a +DA plasmid vector. </p> | ||
| Line 187: | Line 187: | ||
<p>Although conclusive evidence regarding the production of scytonemin was not found, the initial results are promising. Previous studies on algae described a deep brown secretion in cells that were shown to be producing this compound. This pathway has not yet been fully characterized, and additional genes with unknown functions are present within the native <i>N. punctiforme</i> gene cluster. We are currently looking into cloning and expressing these additional genes, and looking for ways to isolate and analyze potential intermediate products within this pathway.</p> | <p>Although conclusive evidence regarding the production of scytonemin was not found, the initial results are promising. Previous studies on algae described a deep brown secretion in cells that were shown to be producing this compound. This pathway has not yet been fully characterized, and additional genes with unknown functions are present within the native <i>N. punctiforme</i> gene cluster. We are currently looking into cloning and expressing these additional genes, and looking for ways to isolate and analyze potential intermediate products within this pathway.</p> | ||
| - | |||
| - | |||
| - | |||
| - | |||
<!---END--> | <!---END--> | ||
Latest revision as of 03:58, 4 October 2012

Like us on FB and follow us on Twitter!
 "
"