Team:Stanford-Brown/HellCell/Desiccation
From 2012.igem.org
(/* Desiccation At a glance Extremophiles: Pantoea agglomerans and Saccharomyces cerevisiae Proteins of interest: choline dehydrogenase and betaine aldehyde dehydrogenase (glycine betaine biosynthesis pathway) and Osmoregulatory trehalose synthe) |
|||
| (6 intermediate revisions not shown) | |||
| Line 22: | Line 22: | ||
| - | ''Saccharomyces cerevisiae'', baker’s yeast, is remarkably resistant to desiccation, and it has been shown that certain osmoprotectant chemicals accumulate in these cells when | + | ''Saccharomyces cerevisiae'', baker’s yeast, is remarkably resistant to desiccation, and it has been shown that certain osmoprotectant chemicals accumulate in these cells when they desiccate. One of these chemicals is trehalose, a disaccharide that was initially implicated in desiccation resistance because of this accumulation (Calahan, Dunham, DeSevo, and Koshland 2011). Surprisingly, attempts to understand trehalose's role and effectiveness in desiccation resistance have produced conflicting and inconsistent results (Calahan et al. 2011). |
| - | + | Glycine betaine (see Cold) also has been found to play an osmoprotectant role in ''E. coli,'' balancing the osmolarity of the cytoplasm when the cell is under osmotic stress (Cayley, Lewis, and Record 1992). | |
| - | + | The gram-negative biocontrol agent ''Pantoea agglomerans'' was ineffective at controlling blue mold on fruits because of its low viability during the long periods of dehydration that it experienced during fruit processing (Bonaterra, Camps, and Montesinos 2005). Its effectiveness was significantly increased when it was engineered to accumulate both trehalose and glycine betaine during desiccation, and it was hypothesized that these osmoprotectants “operate through protection of membrane phospholipids by direct hydrogen bounding with phospolipid head groups maintaining the liquid crystal state and stabilising proteins by water replacement via hydrogen bounding” (Bonaterra, Camps, and Montesinos 2005). | |
| + | |||
| + | To see if the osmoprotectants trehalose and glycine betaine can be applied to conferring desiccation resistance to ''E. coli'', the Hell Cell Squad isolated the genes necessary for biosynthesis of trehalose and glycine betaine from ''E. coli'' and put them into the Test Plasmid (see Test Plasmid). We also tested MntH mutants for desiccation resistance-conferring properties (see Radiation, MntH is also believed to help provide protection against desiccation). | ||
'''Assay''' | '''Assay''' | ||
| - | Liquid cultures of negative control, | + | Liquid cultures of negative control, MntH, betAB, and otsAB transformed NEB5α ''E. coli'' were grown up over night at 37°C. After incubation, a dilution spot assay was conducted on each of the cultures to determine the density of live cells. Next, 15 5cm, round petri dishes were filled with 300uL of negative control bacteria, another 15 with transformants containing MntH, another 15 with transformants containing betAB, and another 15 with transformants containing otsAB. The petri dishes were allowed to desiccate while covered for 24 hours at 37°C while shaking. After the 24 hours period, each plate was resuspended with 1mL of fresh LB. A dilution spot assay was then conducted on each of the petri dishes to determine the final density of live cells. |
| Line 36: | Line 38: | ||
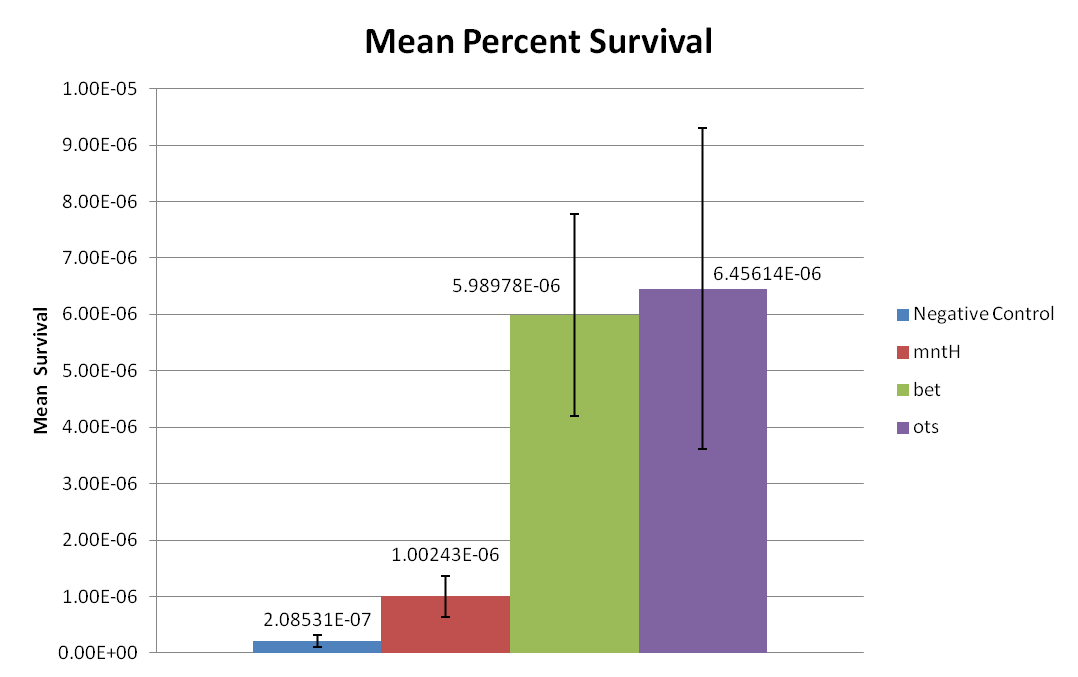
Figure 1: This graph displays the percent of surviving cells after 24 hours of desiccation. The exact survival percentages and standard errors of the mean are displayed above each sample in decimal form. | Figure 1: This graph displays the percent of surviving cells after 24 hours of desiccation. The exact survival percentages and standard errors of the mean are displayed above each sample in decimal form. | ||
| - | [[File: | + | [[File:Desiccation_graph.jpg|800px|center]] |
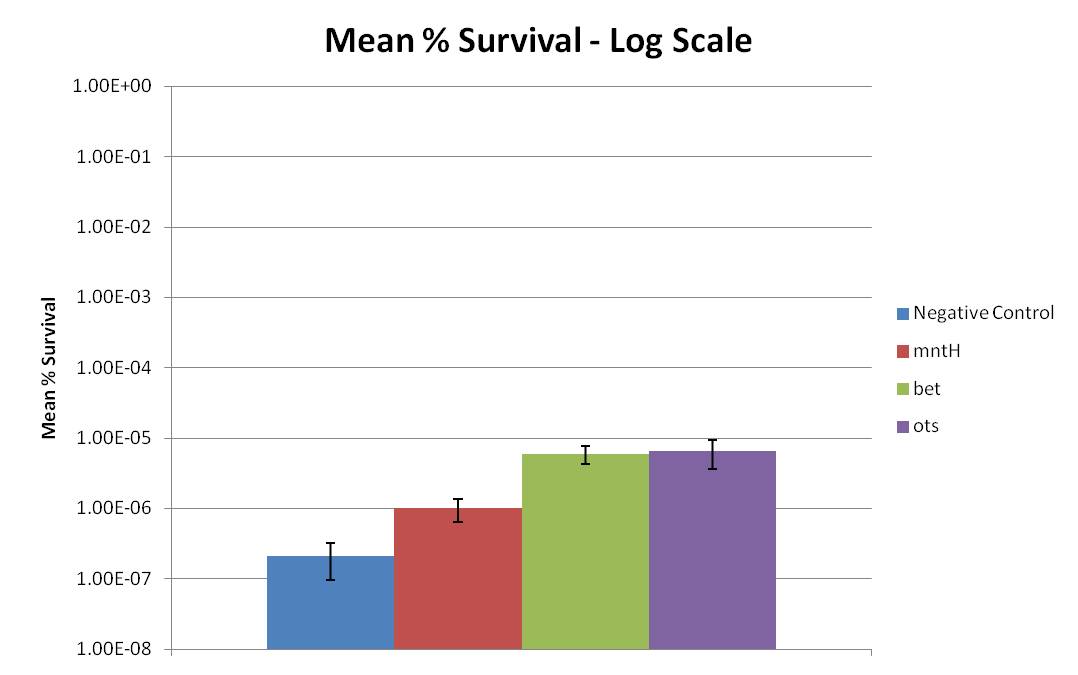
Figure 2: Displays Figure 1 in a log scale. | Figure 2: Displays Figure 1 in a log scale. | ||
| Line 42: | Line 44: | ||
'''Conclusions''' | '''Conclusions''' | ||
| - | Based on cell survival after 24 hours of desiccation, it | + | Based on cell survival after 24 hours of desiccation, it was observed that the bet construct and ots construct provide significant desiccation resistance. Additionally, the MntH construct may also slightly increase desiccation resistance when compared to the negative control, but clearly not to the extent of the bet or ots constructs. This trend can be more clearly observed in Figure 2. Figure 2 clearly displays that the ''E. coli'' MntH construct increases survivability by almost one order of magnitude, while the bet and ots constructs provide almost two orders of magnitude increase in survivability. Thus, all three constructs, Mnth, bet, and ots, provide desiccation resistance. |
Sources: | Sources: | ||
Latest revision as of 02:26, 27 October 2012
Desiccation
At a glance
Extremophiles: Pantoea agglomerans and Saccharomyces cerevisiae
Proteins of interest: choline dehydrogenase and betaine aldehyde dehydrogenase (glycine betaine biosynthesis pathway) and Osmoregulatory trehalose synthesis A and B (trehalose synthesis pathway)
Consensus: Effective
Saccharomyces cerevisiae, baker’s yeast, is remarkably resistant to desiccation, and it has been shown that certain osmoprotectant chemicals accumulate in these cells when they desiccate. One of these chemicals is trehalose, a disaccharide that was initially implicated in desiccation resistance because of this accumulation (Calahan, Dunham, DeSevo, and Koshland 2011). Surprisingly, attempts to understand trehalose's role and effectiveness in desiccation resistance have produced conflicting and inconsistent results (Calahan et al. 2011).
Glycine betaine (see Cold) also has been found to play an osmoprotectant role in E. coli, balancing the osmolarity of the cytoplasm when the cell is under osmotic stress (Cayley, Lewis, and Record 1992).
The gram-negative biocontrol agent Pantoea agglomerans was ineffective at controlling blue mold on fruits because of its low viability during the long periods of dehydration that it experienced during fruit processing (Bonaterra, Camps, and Montesinos 2005). Its effectiveness was significantly increased when it was engineered to accumulate both trehalose and glycine betaine during desiccation, and it was hypothesized that these osmoprotectants “operate through protection of membrane phospholipids by direct hydrogen bounding with phospolipid head groups maintaining the liquid crystal state and stabilising proteins by water replacement via hydrogen bounding” (Bonaterra, Camps, and Montesinos 2005).
To see if the osmoprotectants trehalose and glycine betaine can be applied to conferring desiccation resistance to E. coli, the Hell Cell Squad isolated the genes necessary for biosynthesis of trehalose and glycine betaine from E. coli and put them into the Test Plasmid (see Test Plasmid). We also tested MntH mutants for desiccation resistance-conferring properties (see Radiation, MntH is also believed to help provide protection against desiccation).
Assay
Liquid cultures of negative control, MntH, betAB, and otsAB transformed NEB5α E. coli were grown up over night at 37°C. After incubation, a dilution spot assay was conducted on each of the cultures to determine the density of live cells. Next, 15 5cm, round petri dishes were filled with 300uL of negative control bacteria, another 15 with transformants containing MntH, another 15 with transformants containing betAB, and another 15 with transformants containing otsAB. The petri dishes were allowed to desiccate while covered for 24 hours at 37°C while shaking. After the 24 hours period, each plate was resuspended with 1mL of fresh LB. A dilution spot assay was then conducted on each of the petri dishes to determine the final density of live cells.
Figure 1: This graph displays the percent of surviving cells after 24 hours of desiccation. The exact survival percentages and standard errors of the mean are displayed above each sample in decimal form.
Figure 2: Displays Figure 1 in a log scale.
Conclusions
Based on cell survival after 24 hours of desiccation, it was observed that the bet construct and ots construct provide significant desiccation resistance. Additionally, the MntH construct may also slightly increase desiccation resistance when compared to the negative control, but clearly not to the extent of the bet or ots constructs. This trend can be more clearly observed in Figure 2. Figure 2 clearly displays that the E. coli MntH construct increases survivability by almost one order of magnitude, while the bet and ots constructs provide almost two orders of magnitude increase in survivability. Thus, all three constructs, Mnth, bet, and ots, provide desiccation resistance.
Sources:
Calahan, D., Dunham, M., DeSevo, C., Koshland, D.E. (2011). Genetic analysis of desiccation tolerance in Saccharomyces cerevisiae. Genetics, 189: 507-519.
Bonaterra, A., Camps, J., Montesinos, E. (2005). Osmotically induced trehalose and glycine betaine accumulation improves tolerance to desiccation, survival and efficacy of the postharvest biocontrol agent Pantoea agglomerans EPS125. FEMS Microbiol. Lett., 250: 1-8.
Cayley, S., Lewis, B. A., Record Jr., T. (1992). Origins of the osmoprotective properties of betaine and proline in Escherichia coli K-12. J. Bacteriol. 174(5): 1586-1595.
 "
"