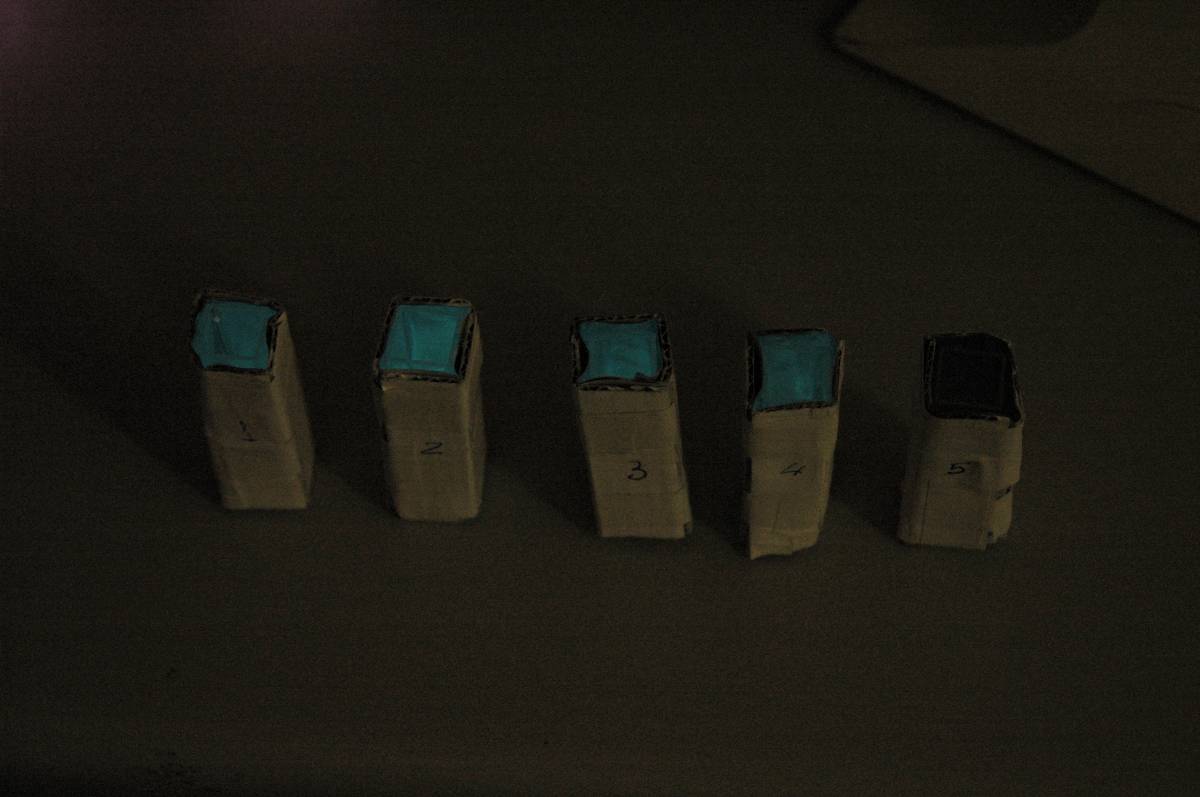Team:Cambridge/Project/Results
From 2012.igem.org
(→Sporage and Distribution) |
Pdmallaband (Talk | contribs) (→Sporage and Distribution) |
||
| (9 intermediate revisions not shown) | |||
| Line 88: | Line 88: | ||
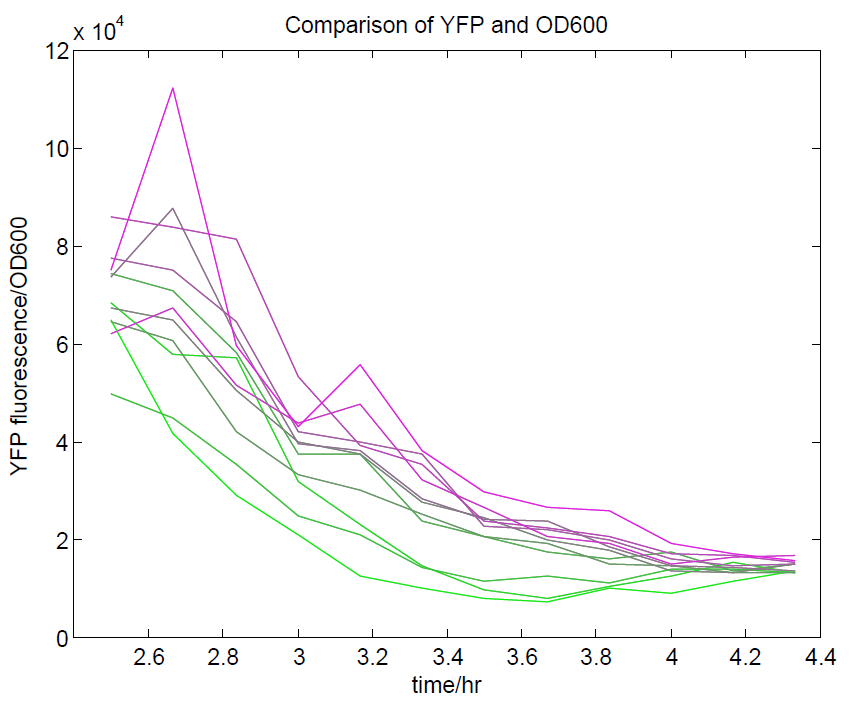
[[File:YFPdividedbyOD600.png|500px|center|thumb|YFP levels divided by OD600. Because YFP should be a measure of cell density along with OD600, we expected that this ratio should approach a constant value. This appears to be the case, although only at higher cell densities.]] | [[File:YFPdividedbyOD600.png|500px|center|thumb|YFP levels divided by OD600. Because YFP should be a measure of cell density along with OD600, we expected that this ratio should approach a constant value. This appears to be the case, although only at higher cell densities.]] | ||
| - | A difference in CFP has also been observed between uninduced and induced (1mM IPTG) plates under the fluorescence microscope. Unfortunately, we were unable to test this induction (and the consequences of ratiometric measurement on the reliability of resulting data) with the plate reader. | + | A difference in CFP has also been observed between uninduced and induced (1mM IPTG) plates under the fluorescence microscope. Unfortunately, we were unable to test this induction (and the consequences of ratiometric measurement on the reliability of resulting data) with the plate reader before the deadline (possibly due to difficulties with culturing the cells in M9 minimal medium). We will reattempt this assay before the jamboree and are confident that results should be as expected. |
| + | |||
| + | Transformation of B. subtilis with the plasmid has also been attempted- unfortunately we are still finetuning our B. subtilis transformation protocol. Again, we hope to show that this works before the Jamboree. | ||
The construct is also sequenced from the beginning and middle (as it is a long part) by Source Bioscience, below is the alignment data from the sequencing (using ClustalW2): | The construct is also sequenced from the beginning and middle (as it is a long part) by Source Bioscience, below is the alignment data from the sequencing (using ClustalW2): | ||
| Line 352: | Line 354: | ||
There is a clear reduction in number of phase-bright spots, both the number decreases over time (see graph, above) and the intensity of remaining spots decreases. This is indicative of spores germinating. | There is a clear reduction in number of phase-bright spots, both the number decreases over time (see graph, above) and the intensity of remaining spots decreases. This is indicative of spores germinating. | ||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| Line 358: | Line 384: | ||
The following shows figures from [[https://2012.igem.org/Team:Cambridge/Project/Sporulation_and_Germination#References <u>Wang et al. 2011</u>]], supporting the spoVA overexpression. | The following shows figures from [[https://2012.igem.org/Team:Cambridge/Project/Sporulation_and_Germination#References <u>Wang et al. 2011</u>]], supporting the spoVA overexpression. | ||
| - | |||
| - | [[ | + | This experiment shows how overexpressed spoVA (strain PS3411) increases the rate of germination. The group isolated individual spores and followed their progress as they germinated using DIC microscopy (high Normalized intensity represent fully sporulated, low Normalized intensity represent fully germinated). |
| + | |||
| + | |||
| + | [[Image:setlow f3a.jpg|200px|left|thumb|Wild Type B.subtilis, germinated on L-alanine at 25°C]] | ||
| + | |||
| + | [[Image:setlow f3c.jpg|200px|right|thumb|strain PS3411 (overexpressed spoVA) B.subtilis, germinated on L-alanine at 25°C]] | ||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | On average spores germinated faster at 45°C for both wild type (WT) and PS3411. Even at 25°C PS4311 still appears to germinate quicker than WT. | ||
| + | |||
| + | Spores were heat shocked at 70°C for 30 minutes prior to measurement. | ||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| - | [[ | + | [[Image:setlow f3d.jpg|200px|left|thumb|Wild Type B.subtilis, germinated on L-alanine at 45°C]] |
| - | [[ | + | [[Image:setlow f3f.jpg|200px|right|thumb|strain PS3411 (overexpressed spoVA) B.subtilis, germinated on L-alanine at 45°C]] |
{{Template:Team:Cambridge/CAM_2012_TEMPLATE_FOOT}} | {{Template:Team:Cambridge/CAM_2012_TEMPLATE_FOOT}} | ||
Latest revision as of 03:56, 27 September 2012


Contents |
Results
Set out below are the developments the team has made over the summer, in tackling our aim and objectives.
RiboSense
Fluoride Riboswitch
Having obtained our DNA, the first test was a rather crude β-Galactosidase assay using cells cultured from the plates Yale sent us, the results of which are shown below, the protocol for which is found here.
These results were very positive as it hinted at a good correlation between fluoride concentration and reporter output.
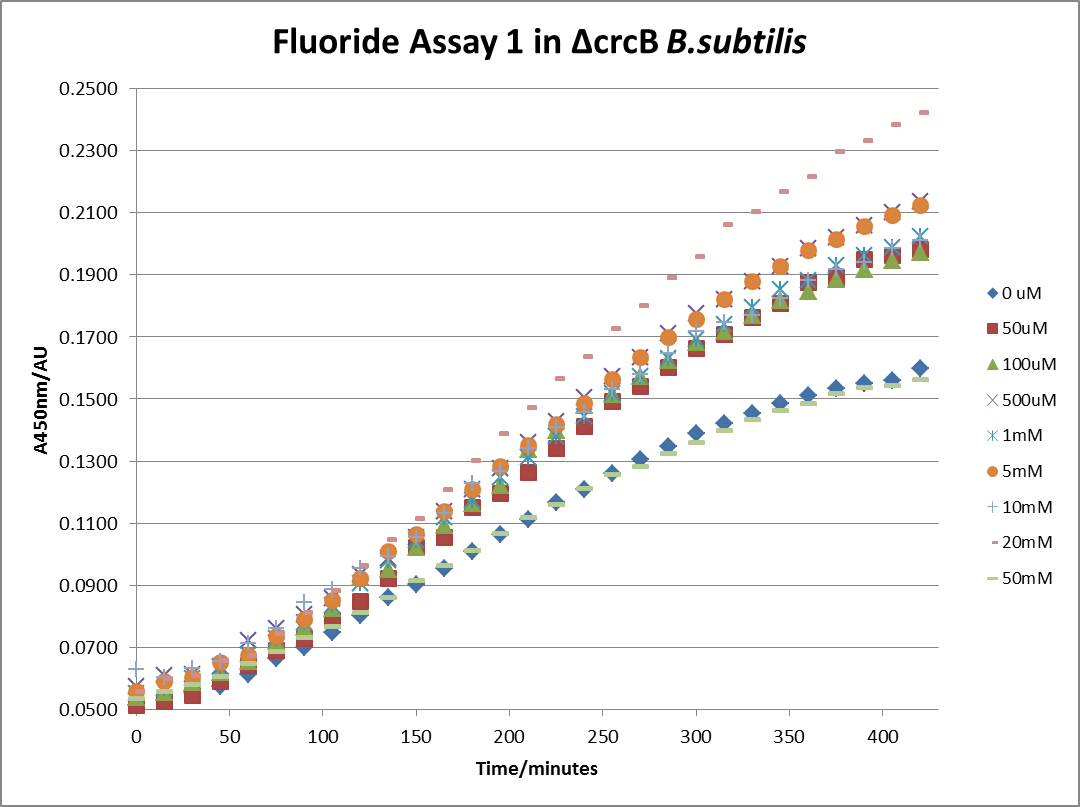
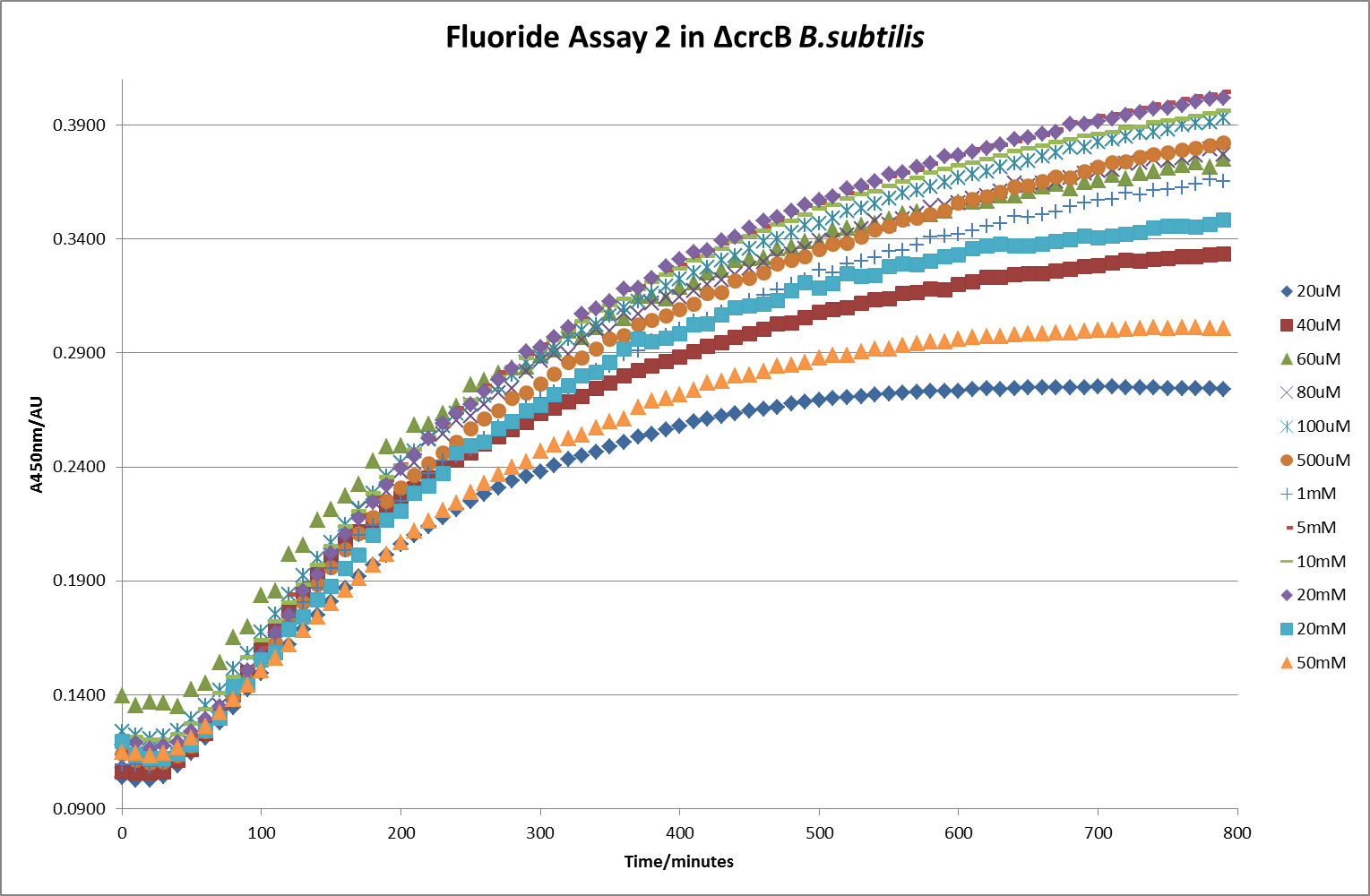
The next step was a more quantitave assay using a 96 well plate reader. This was in line with the assays done in the original paper by Breaker et al. In the paper they carried out a Miller assay, and whilst we have included the full and correct protocol on our protocols page, including the appropriate calculations, we lacked the filters and so were only able to record the A450nm. We feel that this was close enough in wavelength to be a suitable proxy for the A420nm reading required to assay the o-nitrophenol produced. We ran an initial assay and indicative results are below. All the A450 bar charts are based on two repeats, error bars are not included as they would not be statistically significant.
This data seems to show a trend, that at low fluoride concentrations there is a positive relationship between concentration and A450, the A450 then plateaus, and then at high fluoride concentration the A450 drops again, this gives the overall data a bell shape. A possible explanation for this is that there are two conflicting effects, an increase in fluoride leads to an increase in β-galactosidase expression which, in turn, leads to an increased A450. However, the increased fluoride concentrations begin to become toxic and so limit the cell’s ability to produce β-galactosidase, leading to a reduction in A450. It would also seem that the linear region of the initial positive relationship seems to lie between 0μM and 100μM. We therefore repeated the assay, with a longer time course, and with greater resolution in this range, in order to see if the original trend could be reproduced, and also to better quantify this initial correlation. Included below are the results for the repeated assay.
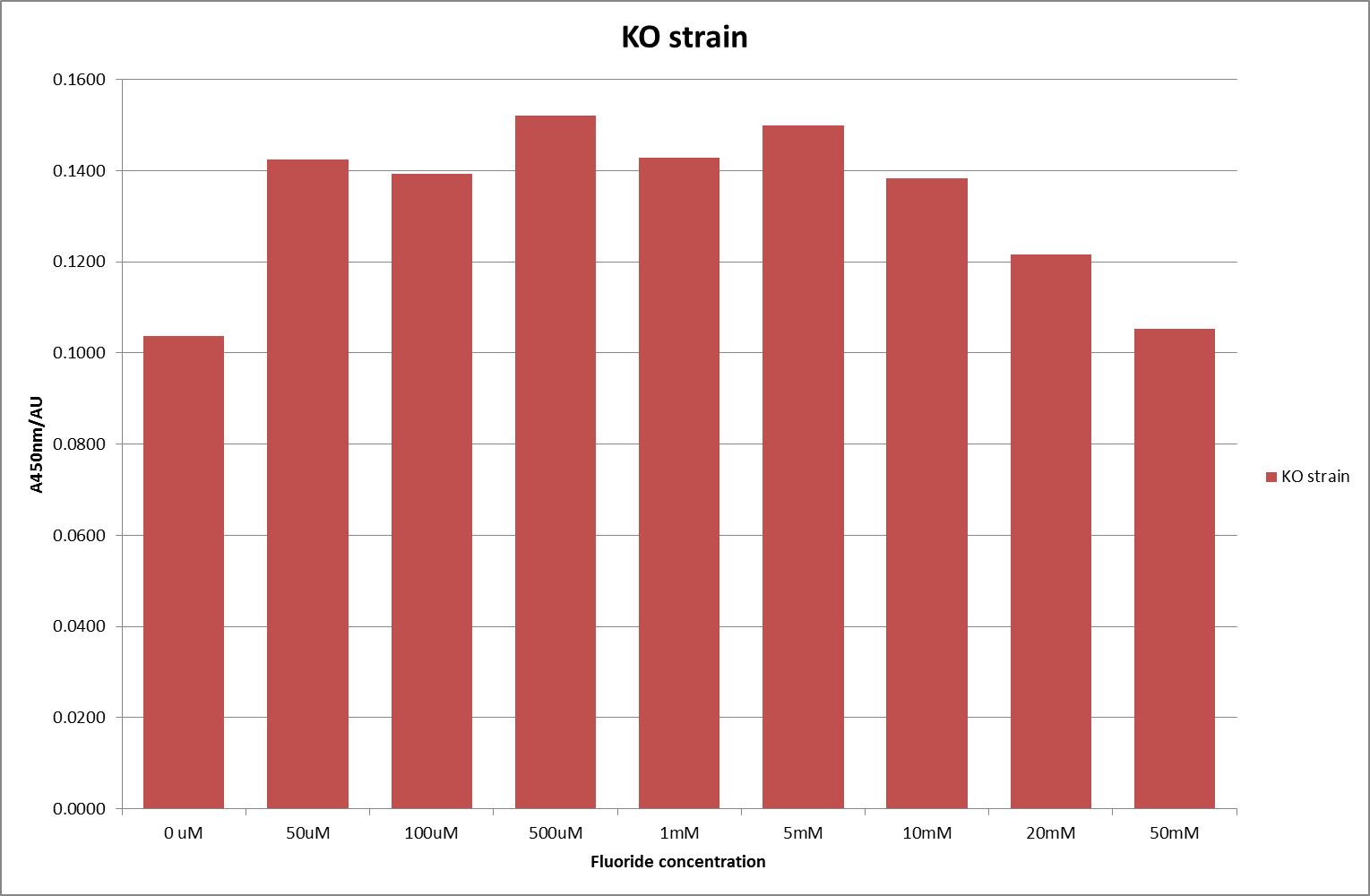
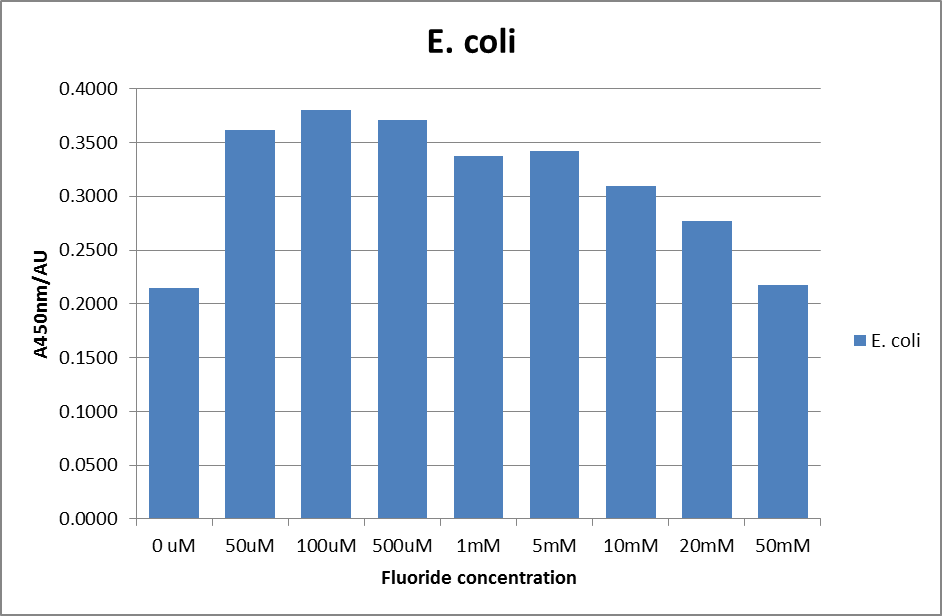
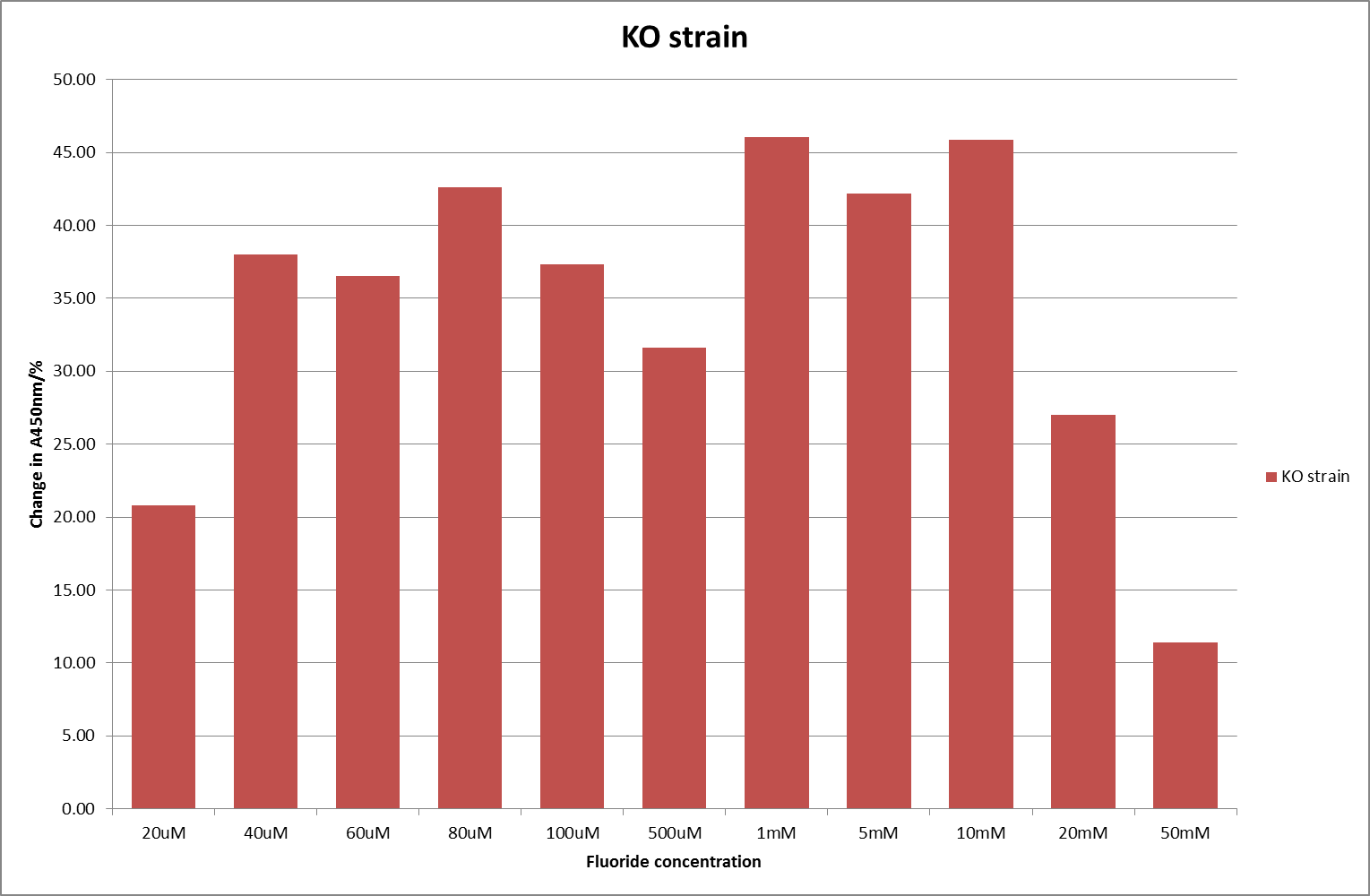
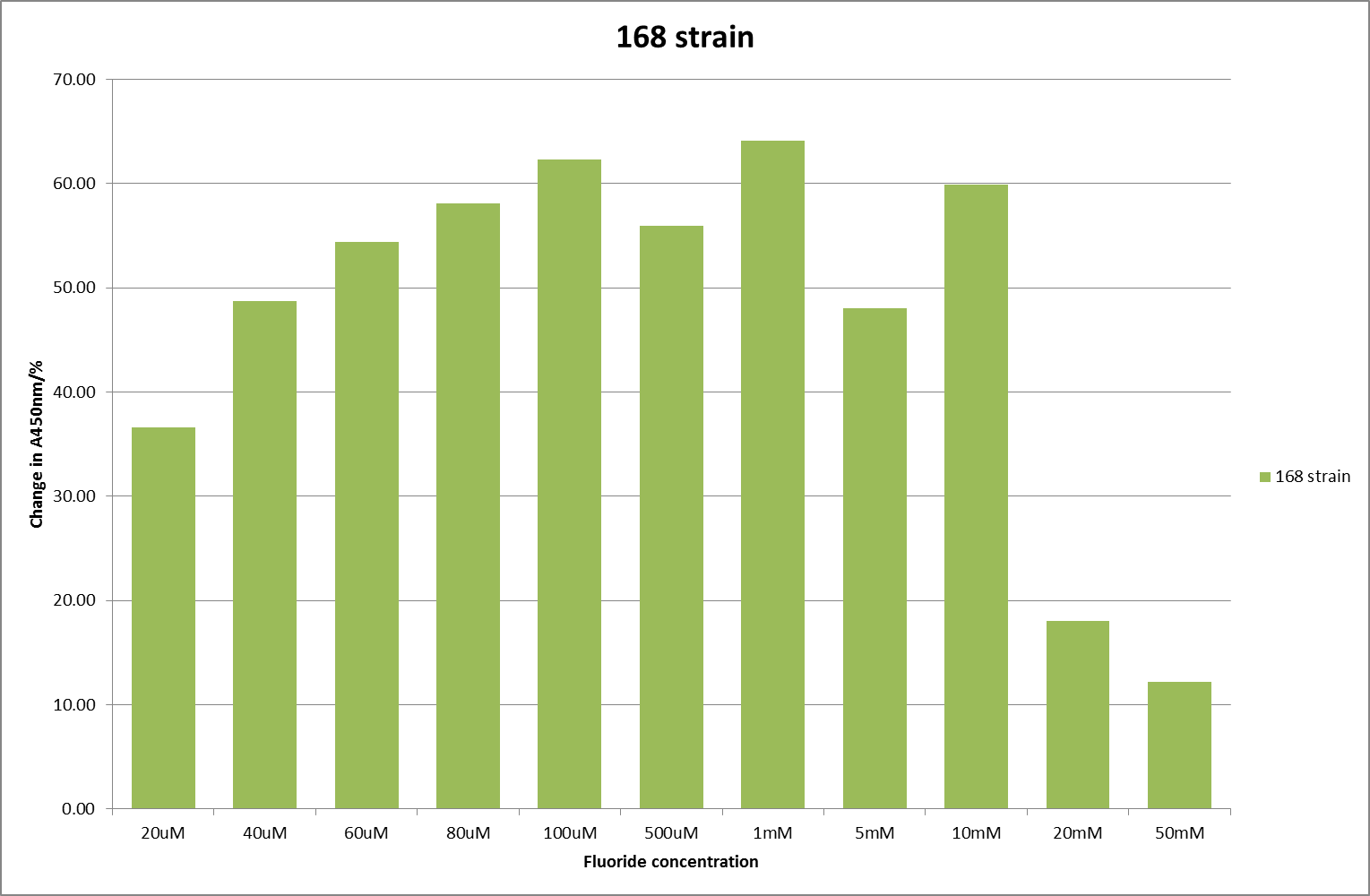
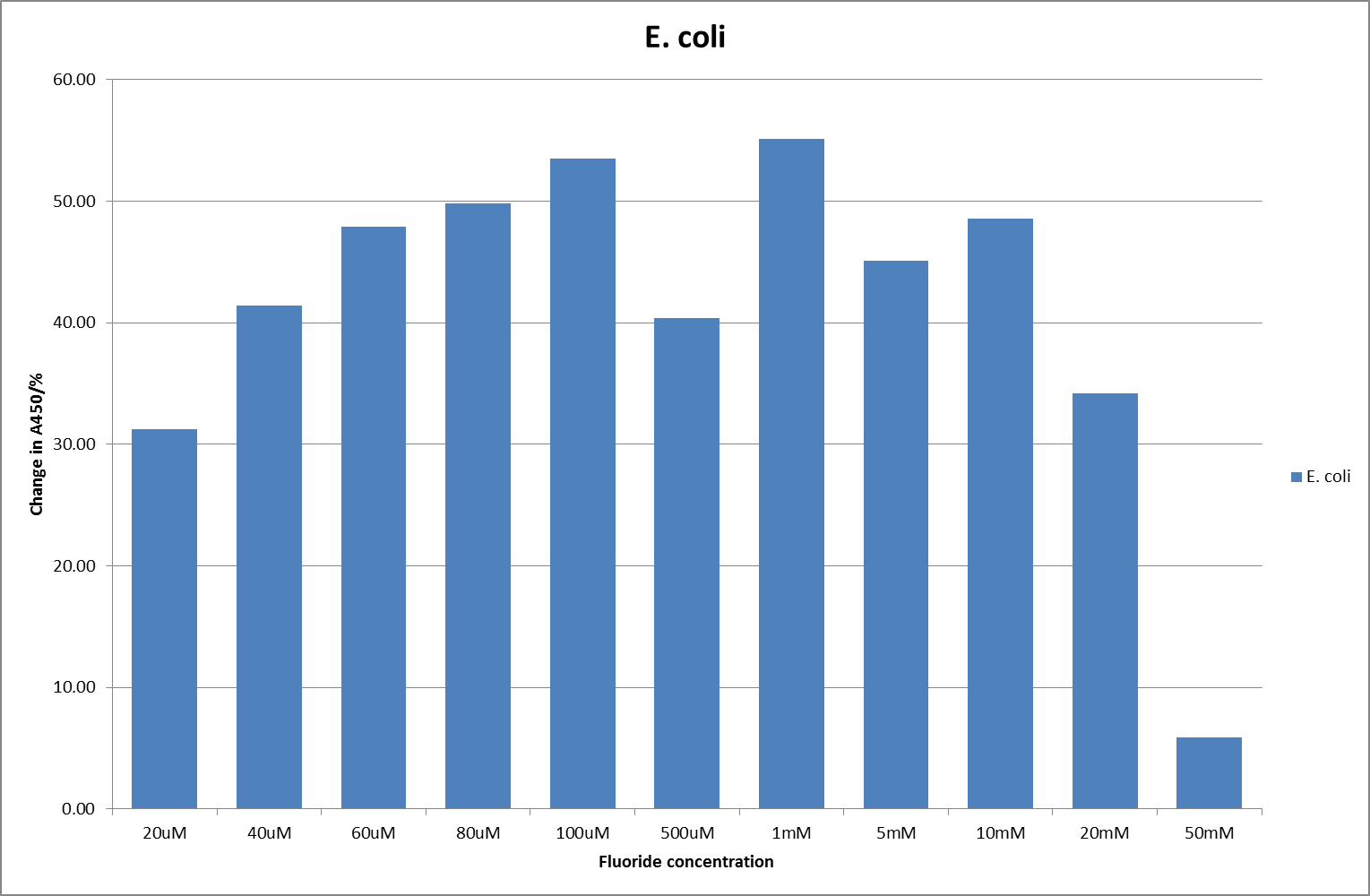
For clarity, and to act as an internal control, the bar chart results are expressed as a percentage change from the same culture, after the same length of time, but with no fluoride added. This assumes that the cultures behaved the same across all fluoride concentrations over the assay, i.e. cell density and productivity were unaffected by the fluoride. This sort of assumption would no longer be needed if a ratiometric system were used, part of our motivation for developing ratiometrica. This data seems to confirm the initial findings, as well as giving greater resolution in the 0μM to 100μM range. The results for higher fluoride concentrations do not seem to quite fit the trend, but as this is consistent across all the cell types it is possible that this was due to errors in making up the solutions, rather than actually being representative of the riboswitch. This data is very encouraging as the WHO safe limit for fluoride is 1.5mg/L (according to [http://dx.doi.org/10.1016/j.envpol.2006.05.007Farooqi et al]) which works out at 79μM. This means our riboswitch is at its most accurate around the safe level for humans. For more information on the implications of this, have a look at our human practices page.
It should be noted that we expected a greater difference in sensitivity between the knockout Bacillus strain provided by Yale, the 168 strain we were using, and the E. coli we used. In the original paper there was a significant, nearly 100-fold, difference in sensitivity between the knock and wild type bacillus strains, but our data did not reproduce this. However, we feel it is actually very positive as it means our part is consistent across multiple chasses, and that it has the desired sensitivity to fluoride, without the need for a special mutant to be ordered. This fits with our goal for our kit to be a standard which is widely compatible.
The next step was to place this construct in a ratiometric fluorescent construct, before eventually testing our luciferase construct with it. But, due to delays in developing the ratiometric construct, we only had one attempt at inserting and testing it and it proved unsuccessful. We shall carry on testing it and, whilst we cannot put our results on here due to the wiki freeze, we hope to present them to you at the jamborees. However, we would like to include here what we plan to do, in order that future teams might benefit. We plan to insert the fluoride riboswitch into the ratiometrica construct and check that they work together as expected, i.e. get consistent readings for various concentrations, in spite of varying cell densities.
Magnesium Riboswitch
For the magnesium riboswitch, we successfully made the following construct:
The sequence of this construct was verified via Sanger sequencing.
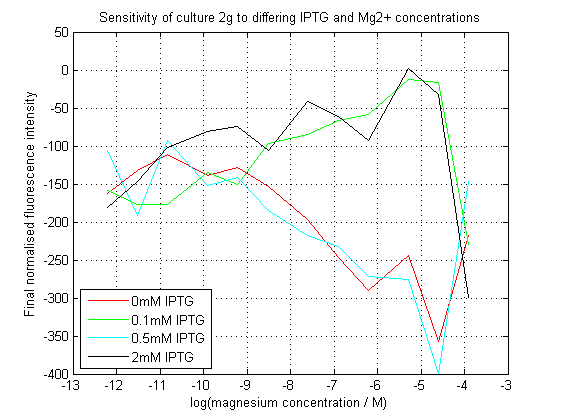
To characterize this construct, we used a 96-well plate reader to assay the effects of the different concentrations of Mg2+ and IPTG on the levels of GFP. We expected the presence of either to allow the expression of sfGFP, however because transcriptional attenuation by the riboswitch occurs before expression of the repressor protein, it was expected that Mg2+ would somehow demonstrate a dominant effect.
As can be seen, our initial assay did not give the expected results. At differing IPTG concentrations, the response to magnesium seems to have inverted.
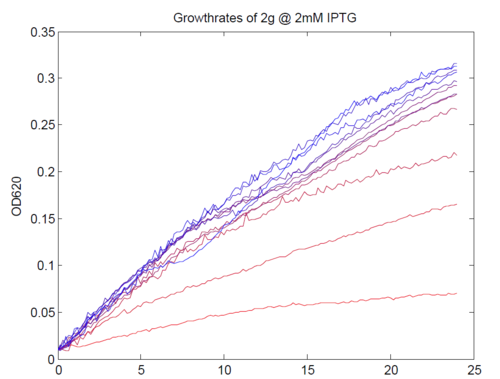
We repeated this experiment, using a different range of magnesium concentrations (1μM - 5mM) and doing duplicates in alternate rows. The cells grew well, although they did not appear to do so in an exponential fashion. It is believed that this may be a feature of the minimal medium in which we were performing the assay, as failed tests using a rich defined medium (which, unfortunately, autofluoresced at GFP wavelengths due to the aromatic amino acids it contained) showed normal exponential growth with the same construct and at identical magnesium concentrations.
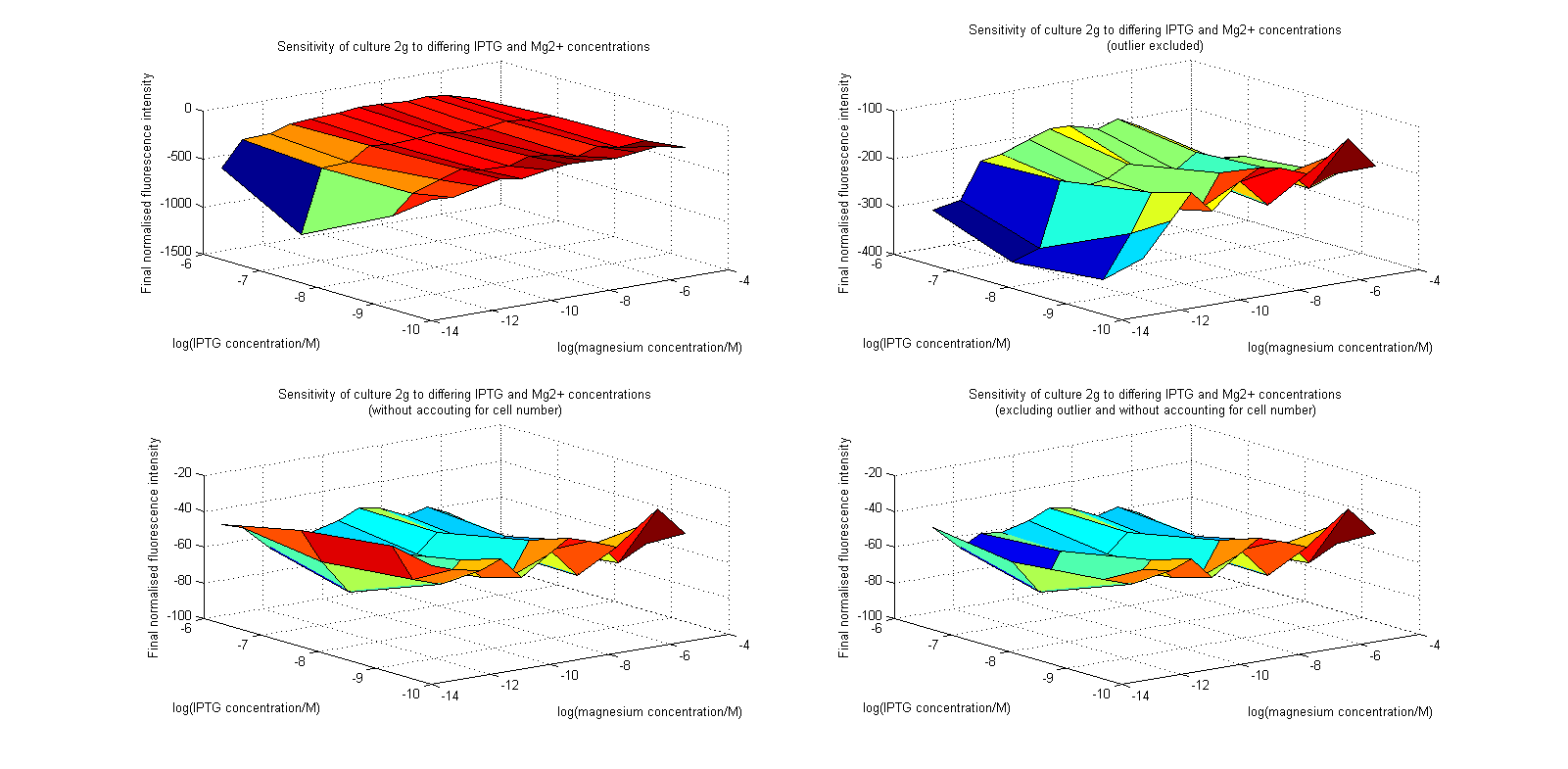
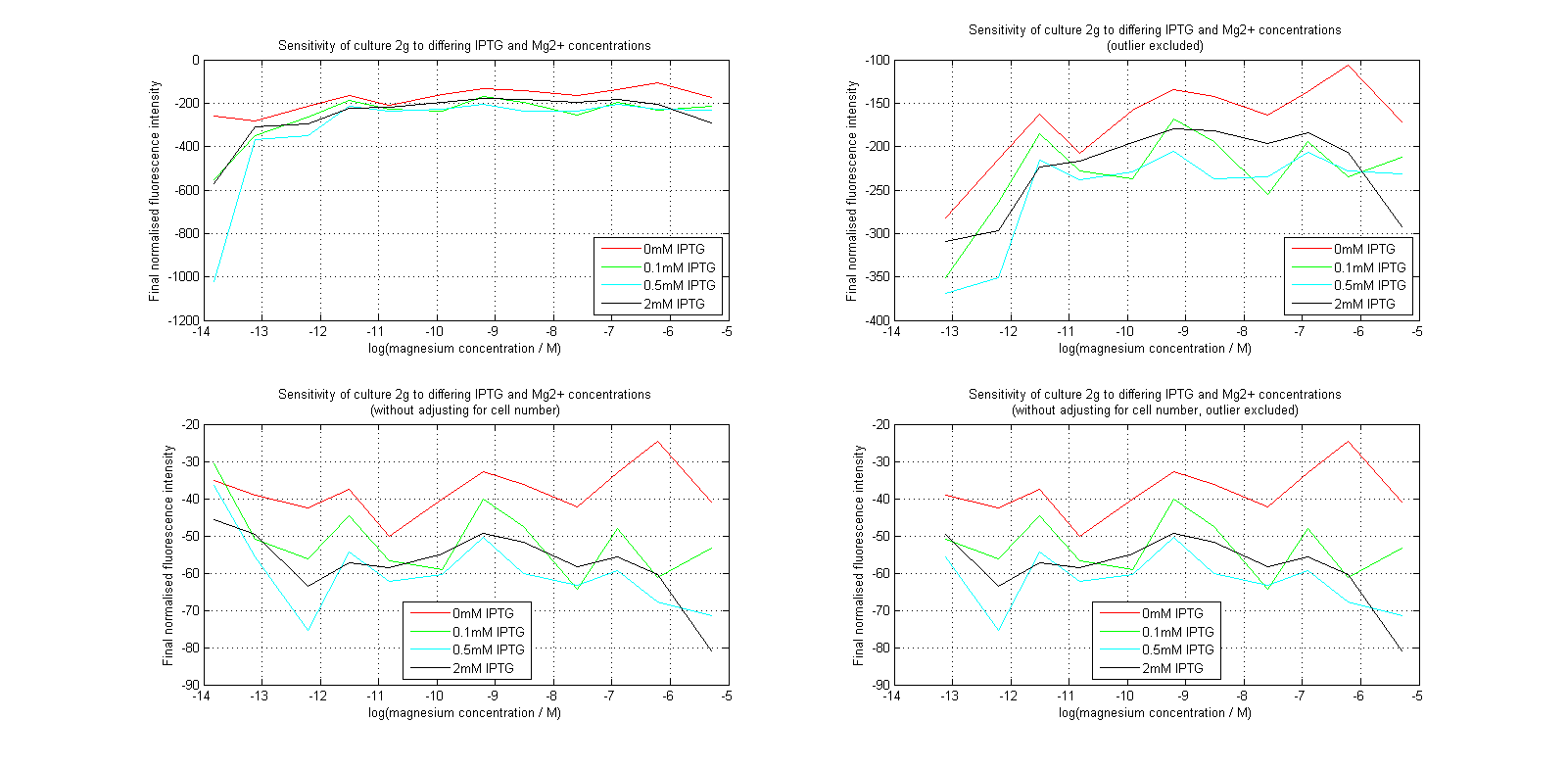
Having collected OD620 data as well as fluorescence data, we hoped to generate graphs containing fluorescence readings normalized to cell density. We used the following formula to normalize our data:
Initial analysis produced fairly convincing data, as shown in the graphs below (top images). Certainly, the part appeared to produce a convincing response to magnesium between 1 μM and ~10μM, within the sensitivity range of the original paper. A more modest increase can be seen past this point. More careful analysis of the raw data indicated that this apparent trend may have been an artifact of the normalization formula, as no particularly convincing trend can be seen in the raw final fluorescence data (bottom images).
However, visual inspection under the fluorescence microscope demonstrated that the low magnesium cultures had very different fluorescent properties, both in quantity and quality of the light produced (higher magnesium cultures had a more yellow hue). We took this as a sign that our plate reader may not be producing reliable data, perhaps not surprising given that we were having to use non-optimal emission and excitation filters. Additionally, the final fluorescence was lower than the initial fluorescence despite the fact that visual inspection demonstrated that it increased considerably from start to finish. This just goes to show the dangers of normalizing data without looking at it first! It also shows the importance of an internal ratiometric control channel which is measured in the same way - this should eliminate the normalization artifacts that we have found from using OD620 as our normalization channel. If the plate reader was unable to measure one fluorescence channel, it is unlikely that it would be able to measure the second, eliminating the artifacts.
We then attempted to transform the construct into Bacillus subtilis, with a view to repeat the same assay in this new chassis. Because the part originally comes from bacillus, we hoped that this might give more useful data. Unfortunately, we did not have time to characterise the part in this chassis, as our transformation attempts failed. This may be an excellent starting point for any future teams seeking to explore this part.
Ratiometrica
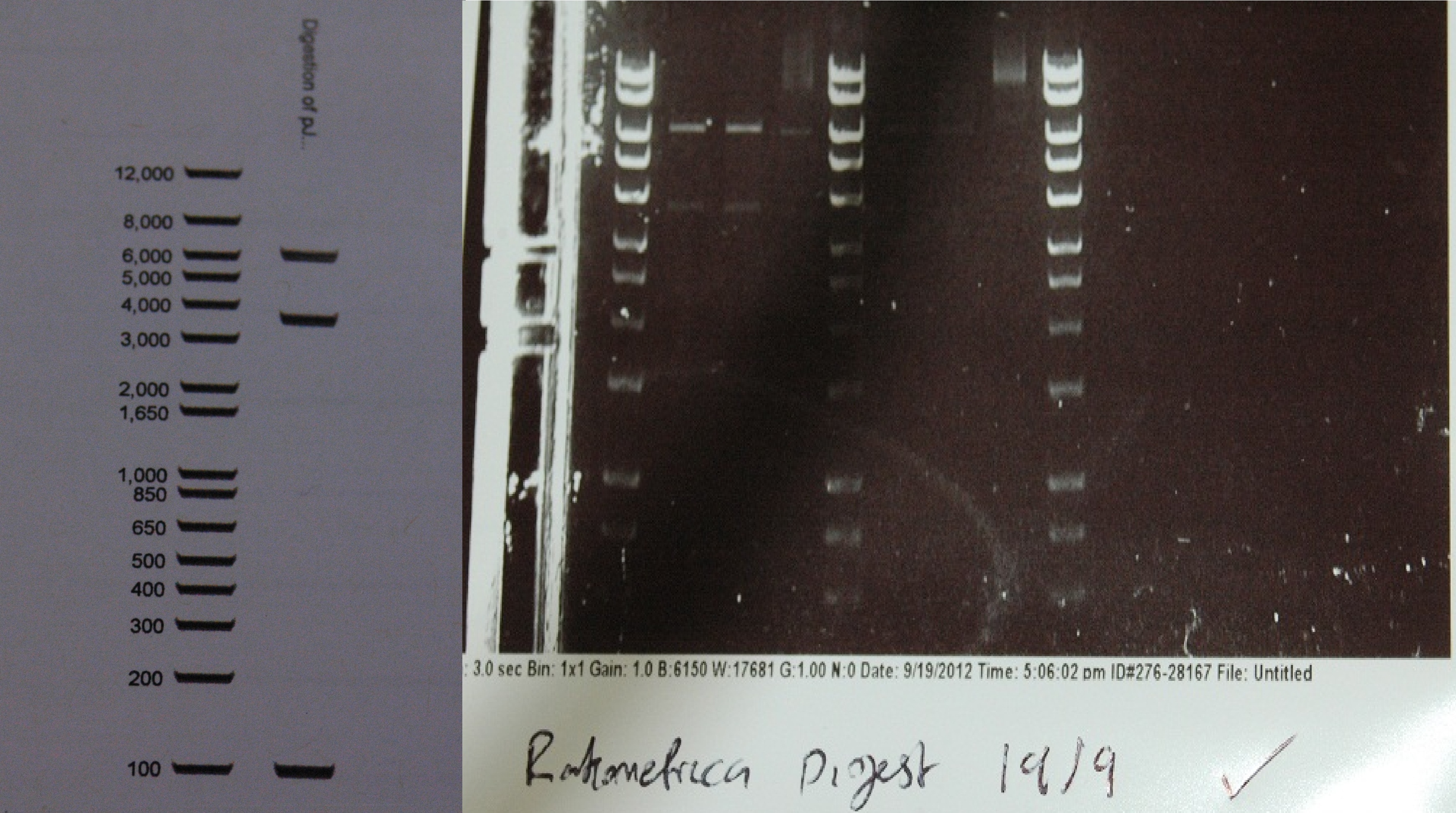
After a lot of technical difficulties, we were able to assemble our fluorescent construct using Gibson assembly. The photo below shows the predicted and obtained digest pattern using HINDIII.
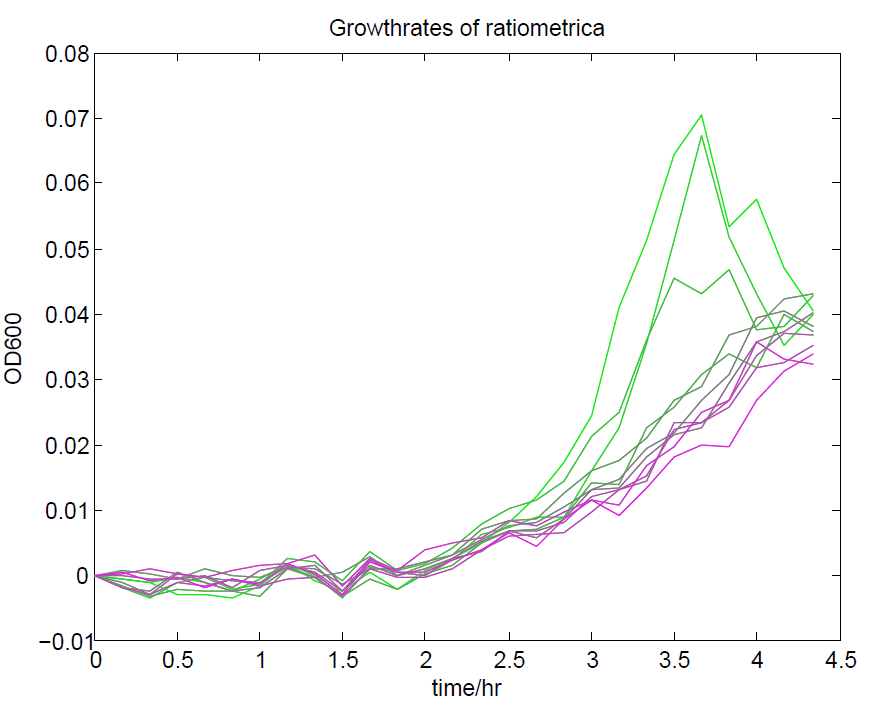
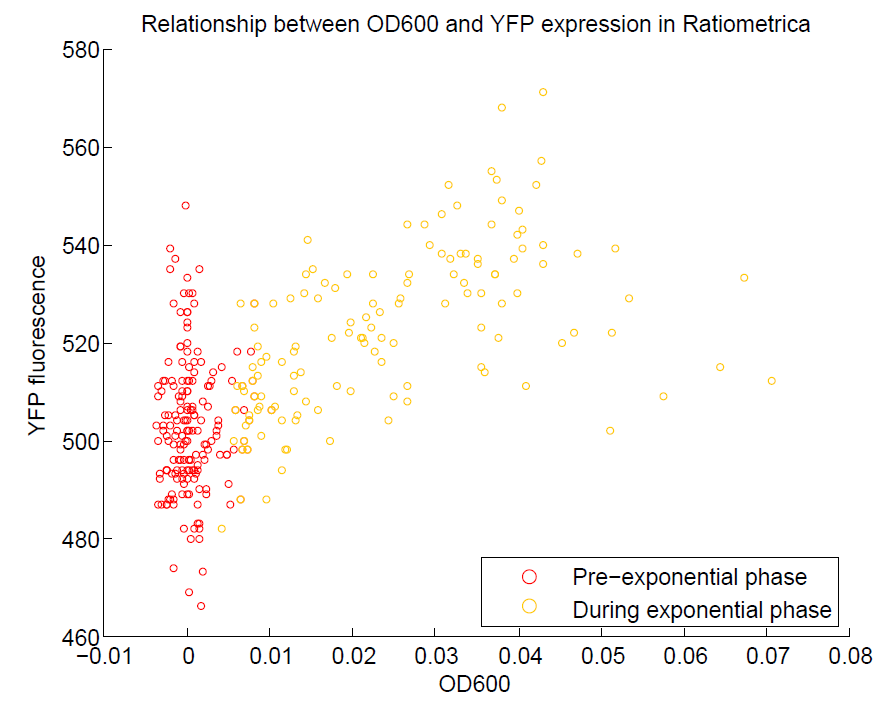
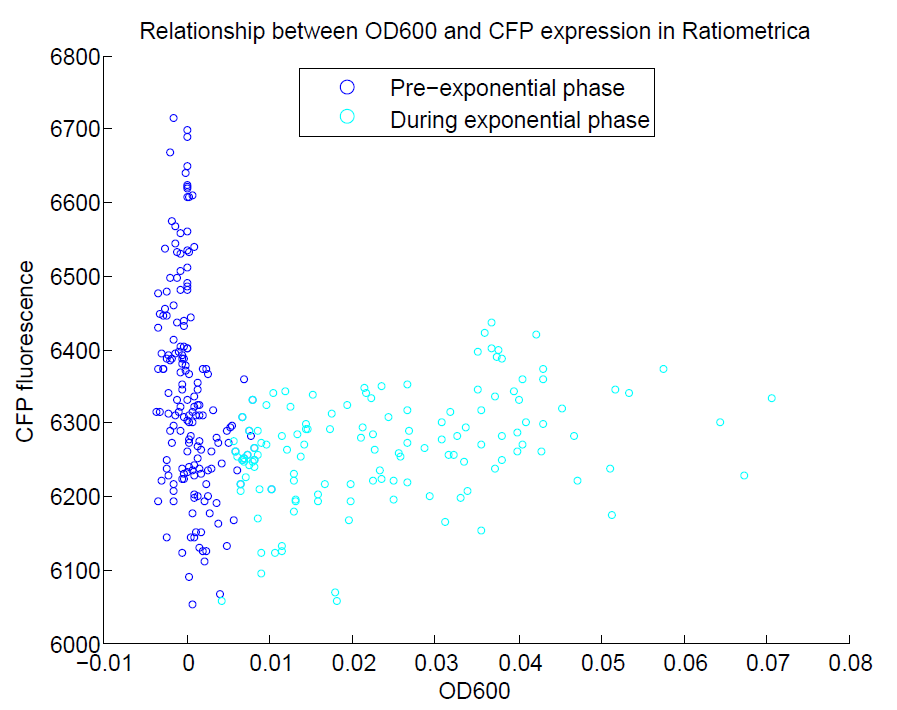
We ran a plate reader assay with ratiometrica with no induction of the reporter CFP. The results of this assay can be seen. In summary:
- No change in CFP is observed with changing OD600.
- YFP change is approximately proportional to OD600.
- The ratio of YFP to OD600 approaches a constant value at higher cell densities.
These properties are exactly what we expected them to be. This assay also tells us that we should aim to take our ratiometric measurements only during late exponential phase, as this is where the data is most reliable.
A difference in CFP has also been observed between uninduced and induced (1mM IPTG) plates under the fluorescence microscope. Unfortunately, we were unable to test this induction (and the consequences of ratiometric measurement on the reliability of resulting data) with the plate reader before the deadline (possibly due to difficulties with culturing the cells in M9 minimal medium). We will reattempt this assay before the jamboree and are confident that results should be as expected.
Transformation of B. subtilis with the plasmid has also been attempted- unfortunately we are still finetuning our B. subtilis transformation protocol. Again, we hope to show that this works before the Jamboree.
The construct is also sequenced from the beginning and middle (as it is a long part) by Source Bioscience, below is the alignment data from the sequencing (using ClustalW2):
CLUSTAL 2.1 multiple sequence alignment
seq ---------------------------NNNNNNNNNNNAANNNNNNNA-- 21
pJS150_seq_-_Ratiometrica AGCCCAGTCCAGACTATTCGGCACTGAAATTATGGGTGAAGTGGTCAAGA 1050
.. ......**..... *
seq ---------------------------------------NNNNNNNTNTN 32
pJS150_seq_-_Ratiometrica CCTCACTAGGCACCTTAAAAATAGCGCACCCTGAAGAAGATTTATTTGAG 1100
... ..*.:.
seq GTN--CNNT----------------------------------------- 39
pJS150_seq_-_Ratiometrica GTAGCCCTTGCCTACCTAGCTTCCAAGAAAGATATCCTAACAGCACAAGA 1150
** * .*
seq -----------TTNNNNTCTN-------------CTAANTNTNAGNGCTC 65
pJS150_seq_-_Ratiometrica GCGGAAAGATGTTTTGTTCTACATCCAGAACAACCTAATTGTGAGCGCTC 1200
**....*** ****.*.*.** ****
seq NNAATTTTTTGNNNAATTNNTNANNNTTTATCTACN-GGTGTGTCAT-AT 113
pJS150_seq_-_Ratiometrica ACAATTTTTTGCAAAAAGTTGTTGACTTTATCTACAAGGTGTGGCATAAT 1250
********* **: .. .:. ********* ****** *** **
seq GTNTGGAANNNCNANNAGCTCACAATTAANGGATGAATTCN-AATGGTGA 162
pJS150_seq_-_Ratiometrica GTGTGGAATTGTGAGCGGCTCACAATTAAAGGAGGAATTCAAAATGGTGA 1300
**.*****... .*. .************ *** ****** ********
seq GCAAGGGCGANGAGCTGTTCACCGGGGTGGNGCCCATCCTGGTCGAGCTG 212
pJS150_seq_-_Ratiometrica GCAAGGGCGAGGAGCTGTTCACCGGGGTGGTGCCCATCCTGGTCGAGCTG 1350
**********.*******************.*******************
seq GACGGCGACGNGANCGGCNACAAGTTCATCGTGTCCTNCGAGGGCGAGGG 262
pJS150_seq_-_Ratiometrica GACGGCGACGTGAACGGCCACAAGTTCAGCGTGTCCGGCGAGGGCGAGGG 1400
**********.** **** ********* ******* .************
seq CGATGCCACCTACGGCAAGCTGACCTTGAAGTTCATCTG-ACCACCGGCA 311
pJS150_seq_-_Ratiometrica CGATGCCACCTACGGCAAGCTGACCCTGAAGTTCATCTGCACCACCGGCA 1450
************************* ************* **********
seq AGCTGCCCGTGCCCTGGCCCACCNTCGTGACCACCCTGACTTGGGGCGTG 361
pJS150_seq_-_Ratiometrica AGCTGCCCGTGCCCTGGCCCACCCTCGTGACCACCCTGACCTGGGGCGTG 1500
*********************** **************** *********
seq CANTGCTTCTNCCGCTACCCCGACCACATGAAGCANCANGACTTCTTCAN 411
pJS150_seq_-_Ratiometrica CAGTGCTTCAGCCGCTACCCCGACCACATGAAGCAGCACGACTTCTTCAA 1550
**.******:.************************.** **********
seq TTCCGCCATGCCCGAAGGCTACTTCNAGNAGCTCACCNTCTTCTTNAAGG 461
pJS150_seq_-_Ratiometrica GTCCGCCATGCCCGAAGGCTACGTCCAGGAGCGCACCATCTTCTTCAAGG 1600
********************* ** **.*** **** ******* ****
seq ACNACGGCAACTACAT--------------TNAN---------------- 481
pJS150_seq_-_Ratiometrica ACGACGGCAACTACAAGACCCGCGCCGAGGTGAAGTTCGAGGGCGACACC 1650
**.************: *.*
seq --------------------------------------------------
pJS150_seq_-_Ratiometrica CTGGTGAACCGCATCGAGCTGAAGGGCATCGACTTCAAGGAGGACGGCAA 1700
CLUSTAL 2.1 multiple sequence alignment
Rat3 -----------------------------NNNNNNNNNNNNNNNNNNTTN 21
pJS150_seq_-_Ratiometrica AGAGTCACACTGGCTCACCTTCGGGTGGGCCTTTCTGCGTTTATAAATTT 2150
... .. .... . **.
Rat3 TGNCNNN-TAATTTTATTGACAACGTCTTATTAACGTTGATATAATTTAA 70
pJS150_seq_-_Ratiometrica TGTCAAAATAATTTTATTGACAACGTCTTATTAACGTTGATATAATTTAA 2200
**.* ******************************************
Rat3 ATTTTATTTGN-NAAAATGGGCTCGTGTTGTACAATAAATGTTACTAGAG 119
pJS150_seq_-_Ratiometrica ATTTTATTTGACAAAAATGGGCTCGTGTTGTACAATAAATGTTACTAGAG 2250
********** *************************************
Rat3 AAAGGTGGTGNATACTAGATGGTGAGCAAGGGCGAGGAGCTGTTCACCGG 169
pJS150_seq_-_Ratiometrica AAAGGTGGTGAATACTAGATGGTGAGCAAGGGCGAGGAGCTGTTCACCGG 2300
********** ***************************************
Rat3 GGTGGTGCCCATCCTGGTCGAGCTGGACGGCGACGTAAACGGCCACAAGT 219
pJS150_seq_-_Ratiometrica GGTGGTGCCCATCCTGGTCGAGCTGGACGGCGACGTAAACGGCCACAAGT 2350
**************************************************
Rat3 TCAGCGTGTCCGGCGAGGGCGAGGGCGATGCCACCTACGGCAAGCTGACC 269
pJS150_seq_-_Ratiometrica TCAGCGTGTCCGGCGAGGGCGAGGGCGATGCCACCTACGGCAAGCTGACC 2400
**************************************************
Rat3 CTGAAGTTCATCTGCACCACCGGCAAGCTGCCCGTGCCCTGGCCCACCCT 319
pJS150_seq_-_Ratiometrica CTGAAGTTCATCTGCACCACCGGCAAGCTGCCCGTGCCCTGGCCCACCCT 2450
**************************************************
Rat3 CGTGACCACCTTCGGCTACGGCCTGCAATGCTTCGCCCGCTACCCCGACC 369
pJS150_seq_-_Ratiometrica CGTGACCACCTTCGGCTACGGCCTGCAATGCTTCGCCCGCTACCCCGACC 2500
**************************************************
Rat3 ACATGAAGCTGCACGACTTCTTCAAGTCCGCCATGCCCGAAGGCTACGTC 419
pJS150_seq_-_Ratiometrica ACATGAAGCTGCACGACTTCTTCAAGTCCGCCATGCCCGAAGGCTACGTC 2550
**************************************************
Rat3 CAGGAGCGCACCATCTTCTTCAAGGACGACGGCAACTACAAGACCCGCGC 469
pJS150_seq_-_Ratiometrica CAGGAGCGCACCATCTTCTTCAAGGACGACGGCAACTACAAGACCCGCGC 2600
**************************************************
Rat3 CGAGGTGAAGTTCGAGGGCGACACCCTGGTGAACCGCATCGAGCTGAAGG 519
pJS150_seq_-_Ratiometrica CGAGGTGAAGTTCGAGGGCGACACCCTGGTGAACCGCATCGAGCTGAAGG 2650
**************************************************
Rat3 GCATCGACTTCAAGGAGGACGGCAACATCCTGGGGCACAAGCTGGAGTAC 569
pJS150_seq_-_Ratiometrica GCATCGACTTCAAGGAGGACGGCAACATCCTGGGGCACAAGCTGGAGTAC 2700
**************************************************
Rat3 AACTACAACAGCCACAACGTCTATATCATGGCCGACAAGCAGAAGAACGG 619
pJS150_seq_-_Ratiometrica AACTACAACAGCCACAACGTCTATATCATGGCCGACAAGCAGAAGAACGG 2750
**************************************************
Rat3 CATCAAGGTGAACTTCAAGATCCGCCACAACATCGAGGACGGCAGCGTGC 669
pJS150_seq_-_Ratiometrica CATCAAGGTGAACTTCAAGATCCGCCACAACATCGAGGACGGCAGCGTGC 2800
**************************************************
Rat3 AGCTCGCCGACCACTACCAGCAGAACACCCCCATCGGCGACGGCCCCGTG 719
pJS150_seq_-_Ratiometrica AGCTCGCCGACCACTACCAGCAGAACACCCCCATCGGCGACGGCCCCGTG 2850
**************************************************
Rat3 CTGCTGCCCGACAACCACTACCTGAGCTACCAGTCCGCCCTGAGCAAAGA 769
pJS150_seq_-_Ratiometrica CTGCTGCCCGACAACCACTACCTGAGCTACCAGTCCGCCCTGAGCAAAGA 2900
**************************************************
Rat3 CCCCNAACGAGAAGCGCGATCACATGGTCCTGCTGGAGTTCGTGACCGCC 819
pJS150_seq_-_Ratiometrica CCCC-AACGAGAAGCGCGATCACATGGTCCTGCTGGAGTTCGTGACCGCC 2949
**** *********************************************
Rat3 GCCGGGATCACTCTCGGCATGGACGAGCTGTACAAGTAATAATGATACTA 869
pJS150_seq_-_Ratiometrica GCCGGGATCACTCTCGGCATGGACGAGCTGTACAAGTAATAATGATACTA 2999
**************************************************
Rat3 GAGCCAGGCATCAAATAAAACGAAAGGCTCAGTCGAAAGACTGGGCCTTT 919
pJS150_seq_-_Ratiometrica GAGCCAGGCATCAAATAAAACGAAAGGCTCAGTCGAAAGACTGGGCCTTT 3049
**************************************************
Rat3 CGTTTTATCTGTTGTTTGTCGGTGAACGCTCTCTACTAGTCATCATTTCC 969
pJS150_seq_-_Ratiometrica CGTTTTATCTGTTGTTTGTCGGTGAACGCTCTCTACTAGTCATCATTTCC 3099
**************************************************
Rat3 TTCCGAAAAAACGGTTGCATTTAAATCTTACATATGTAATACTTTTCAAA 1019
pJS150_seq_-_Ratiometrica TTCCGAAAAAACGGTTGCATTTAAATCTTACATATGTAATACTTT-CAAA 3148
********************************************* ****
Rat3 GACTACATTTTGTAAGATTTGATGTTTGAANNCGGGCTGAAANATCGGTA 1069
pJS150_seq_-_Ratiometrica GACTACATTT-GTAAGATTTGATGTTTGAG-TCGG-CTGAAAGATCG-TA 3194
********** ******************. .*** ******.**** **
Rat3 CGTACCCNNNNTTGTTTCNNNATNNNNCAG-CCNATNNNCTNNNNGNATA 1118
pJS150_seq_-_Ratiometrica CGTACCAATTATTGTTTCGTGATTGTTCAAGCCATAACACTGTAGGGATA 3244
******. .. *******...**....**. ** :: **.. .*.***
Rat3 NNNNNNNAAGAGNNCTTCNNCNGGNNACNANTCANNNAANNANTNAANCN 1168
pJS150_seq_-_Ratiometrica GTGG--AAAGAGTGCTTCATCTGGTTACGA-TCAATCAAATATTCAAACG 3291
.... *****..**** .*.**..**.* *** . ** .*.* ** *.
Rat3 GNNGGNNNACNNNNTNNNN---ANNNNNNANNTTNNNCGAANNN------ 1209
pJS150_seq_-_Ratiometrica GAGGGAG-ACGATTTTGATGAAACCAGTAACGTTATACGATGTCGCAGAG 3340
* .** . **. ..*.. . * .. * .** . ***:..
Rat3 ----CCNNNNNNNNTNNNNNNNNGNNNNNNNNNANNN------------- 1242
pJS150_seq_-_Ratiometrica TATGCCGGTGTCTCTTATCAGACCGTTTCCCGCGTGGTGAACCAGGCCAG 3390
**..... . *. . . .... . ....
We are drafting an RFC with the aim to use this construct as a new standard to characterise promoters, both constitutive and inducible, in E. coli and B. subtilis.
Our ratiometric luciferase construct arrived with full sequence coverage. Unfortunately, during construction it was found to be unexpectedly toxic. This hampered characterisation, as it the construct tended to be lost, and necessitated its submission to the registry in a low copy number backbone (with permission from HQ).
Starting from the ground up, we first showed that the part was constitutively luminescing. We also observed that the colonies were orange. This was not initially expected, however this is probably because we designed the construct with consensus RBSes, so any leaky transcription through the inducible promoter would result in enough protein to be visible. We showed that orange colour and luminescence cosegregated, implying that the entire construct is being lost, not just part of it.
Given that IPTG induction seemed unnecessary for production of mOrange, we reasoned that any spectral shift in the emission of the luciferase should be visible without induction. Our next move was to see if we could detect a difference with the filters we used for the instrumentation.
This was done at quite a late stage and in a slightly impromptu fashion. The photographs above directly compare the normal lux biobrick (BBa_K325909) on the left, and our construct on the right, with the same filter. There does seem to be a difference in the quantity of light coming through the filter. However it could be due to colony density (although they seem to be of similar intensities without the filter). Interesting though these results are, they are not quantitative or well-controlled enough to constitute confirmation that the luxA-mOr fusion is behaving as expected, rather they are an indication that it may be.
To categorically confirm that the spectrum is as we expected we would want to fully characterise the emission spectrum with a scanning luminometer. We do not currently have access to a scanning luminometer in time to submit the data here before the wiki freeze, however we hope to obtain this data in the next week, and to be able to present it at the jamboree.
Instrumentation (Biologger)
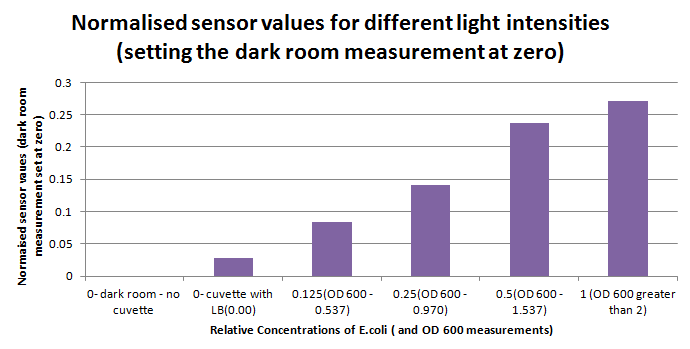
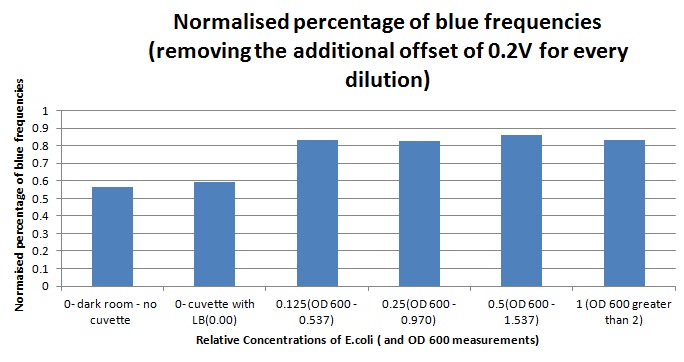
Having our instrumentation completed, as can be seen in our Instrumentation (Biologger) page, the sensitivity of the sensor placed in the right position was tested using a dilution series of luciferase-producing E.coli. 20ml Cultures were grown overnight from single colonies. The cultures were induced with 40ul of 1.5M arabinose (for a final concentration of 3mM). Cultures were left for 2 1/2 hours for full induction. Subsequently, a culture was pelleted and resuspended in 4ml LB. Doubling dilutions, of volume 2ml, were made from this concentrate, down to 1/8th concentration. 1ml of each 2ml dilution was analysed in each cuvette, which was placed in the cuvette holder we made ourselves. The result was very good. An almost linear relationship was obtained when data were normalised with the sensor value taken in the dark room (the latter set at zero) without using the cuvette holder (1-(sensor value/sensor value in absolute dark)), presenting the sensitivity of the sensor to different intensities of light. This behaviour was expected due to the changing offset affecting the luciferase spectrum curve at different light intensities. The offset, using our data, was calculated to be about 0.2V for each dilution. A second graph is shown which takes into account this offset (and removes it), thus showing the presence of blue frequencies. The result was as expected, as the presence of blue frequencies throughout the dilution series is not only detected, but also found to be approximately constant. The raw data of this investigation can be found in the Lab book.
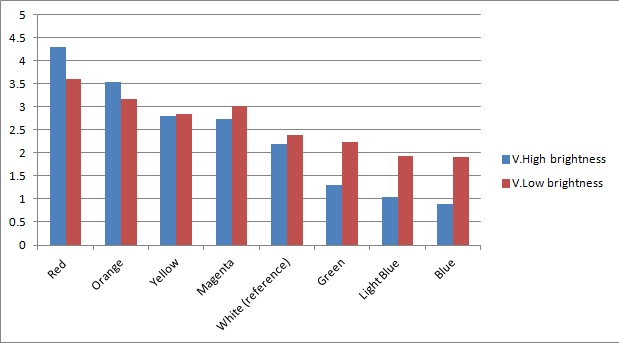
Once the sensor was tested for sensitivity, we tested that our circuitry correctly identified different frequencies (colours) of light. As can be seen below, measurements taken from orange and blue light yield values respectively above and below those from white light (our reference point). The data was taken using constant intensity of light for each case (V.High and V.Low brightness, as specified in the application). This was done with the aid of an Android phone and a specialised software application, called Color Flashlight, downloaded from the official Market.
As expected from the potential-divider design of our circuitry, orange and red frequencies caused the resistance of the LDR with the orange filter to decrease, leading to a higher voltage across the LDR with the blue filter. The opposite effect was observed with blue light. The reason that the white reference point is a bit lower than 2.5V (the expected value for a non-biased circuitry with a 5V source), is because we use resistors of total net resistance 1.67 kΩ before the blue LDR. This was done to bias the circuitry towards blue (i.e. decreasing the starting value, thus the sensor identifies always a bit more blue - this can be shown in previous graphs as well) and thus cause orange light to have a larger impact when present. This was used in an attempt to compensate for the fact that the peak at 560nm (Orange) in MOrange/luciferase fusion spectrum is lower than the one at 490 nm (Blue). Even though we did not manage to test the latter with transformed bacteria, the data collected in all the previous experiments makes us confident that the instrumentation is at least adequately functional.
As the major part of the instrumentation, the bio-electronic interface, had been made and tested, now we turned to testing the other parts of our deveoped kit. This included the mechanical chassis of the prototype, the electronic/mechatronic (sensory and motory) components, and of course the software. The overview of the finished hardware/software can be seen in our Intstrumentation (Biologger) page. Below, the videos showing our instrumentation in action can be seen.
Sporage and Distribution
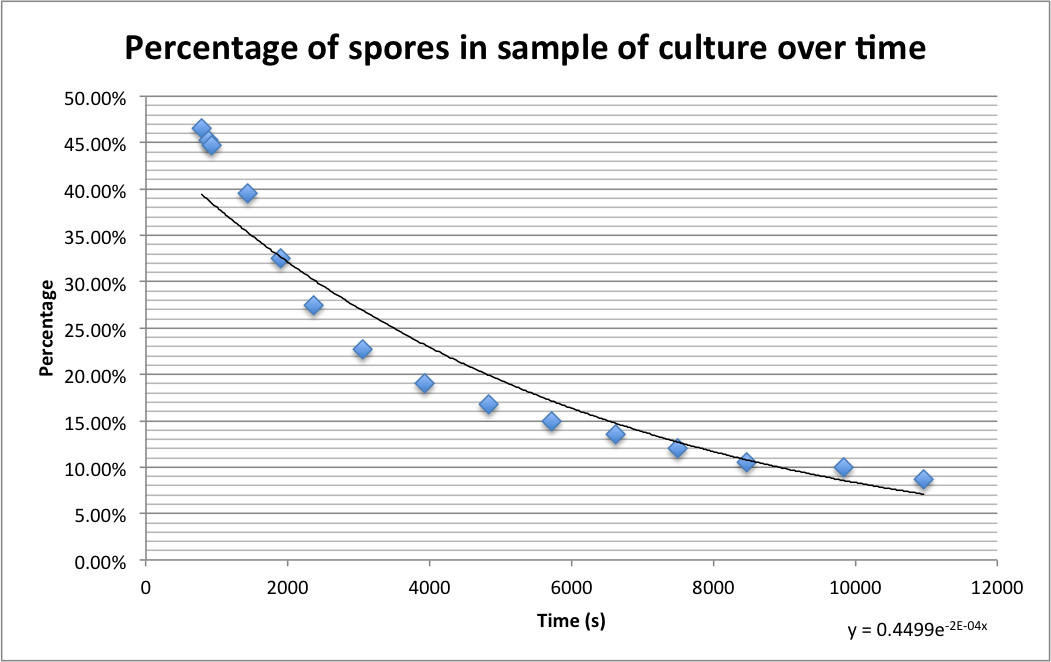
Please find detailed explanations of this part of the project on our Sporulation and Germination main page and on our Design Process page The graph below shows the percentage of spores (strain PS3411) counted in a sample of culture that were pipetted onto germination medium containing agarose pads. The graph shows the spores germinating over time.
The images below show a time lapse taken using DIC microscopy from which the time point values were calculated. Using excel's exponential line fitting, the half life of these spores is calculated to be 5000 seconds (83 minutes). This is much slower than was hoped. However, we were unable to replicate the conditions used by Prof. Setlow in the papers linked on the attributions page due to limitations of equipment in our lab (e.g. no access to a mounted stage or difco sporulation medium).
Furthermore, we were unsuccessful (three times!) in making spores from the wild type Bacillus 168 strain and are unable to explain this. Therefore we haven't provided comparative data to prove that there is an increase in germination rate when the fast promoter is swapped in.
In the following microscopy images and animation, spores show up as phase-bright spots whereas vegetative cells are dark.

There is a clear reduction in number of phase-bright spots, both the number decreases over time (see graph, above) and the intensity of remaining spots decreases. This is indicative of spores germinating.
Published data
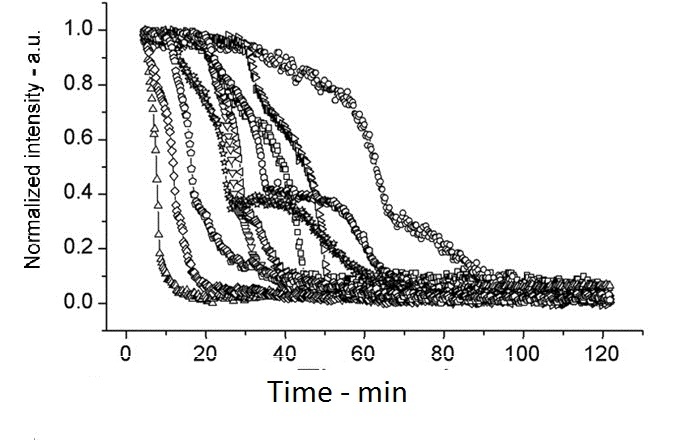
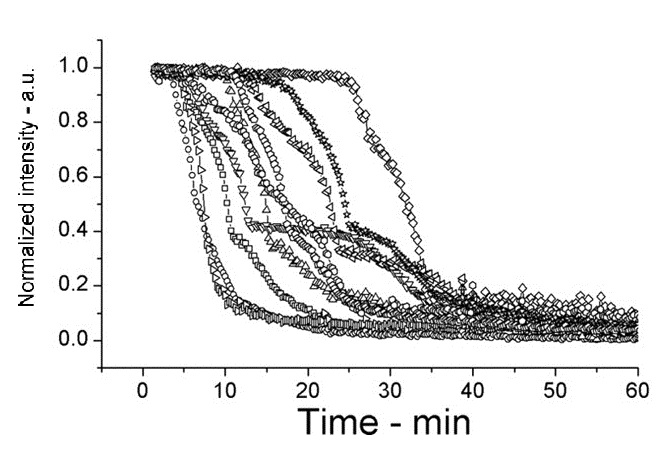
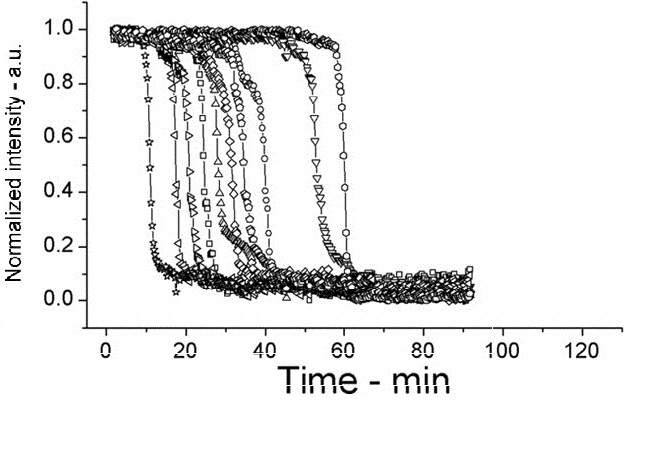
The following shows figures from [Wang et al. 2011], supporting the spoVA overexpression.
This experiment shows how overexpressed spoVA (strain PS3411) increases the rate of germination. The group isolated individual spores and followed their progress as they germinated using DIC microscopy (high Normalized intensity represent fully sporulated, low Normalized intensity represent fully germinated).
On average spores germinated faster at 45°C for both wild type (WT) and PS3411. Even at 25°C PS4311 still appears to germinate quicker than WT.
Spores were heat shocked at 70°C for 30 minutes prior to measurement.
 "
"