Team:Colombia/Modeling/Results
From 2012.igem.org
(→Ralstonia:) |
(→Rust:) |
||
| (32 intermediate revisions not shown) | |||
| Line 9: | Line 9: | ||
| - | The mathematical model should help the experimental design to optimize the circuit. This case was not the exception. The | + | The mathematical model should help the experimental design to optimize the circuit. This case was not the exception. The figure below shows the original design of the circuit. |
<html> | <html> | ||
| Line 16: | Line 16: | ||
</html> | </html> | ||
| - | [[File: | + | [[File:toget.png|600px|thumb|center|Figure 1. Our desing before modeling results]] |
| - | + | ||
| - | + | ||
| - | + | ||
<html> | <html> | ||
| Line 26: | Line 23: | ||
</html> | </html> | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | + | Once results from simulations were obtained ,we had to make little changes to the proposed system: | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | + | 1. As it is shown, before results of simulations, there was an unknown promoter for LuxR. In the beginning, it was established that this promoter must had been constitutive. From simulations results we conclude that response curves of the system were not consistent with the reality (LuxR had giant concentrations and the salicylic acid never went up). In the designed system, LuxR had to interact with LuxI and turn on the response. Thus, it was thought that LuxR must had been in a similar concentration of LuxI. Consequently, LuxI was putted under the same promoter of LuxR, hence the two proteins will be promoted at the same time. | |
| - | + | ||
| - | + | ||
| - | + | ||
<html> | <html> | ||
| Line 51: | Line 34: | ||
</html> | </html> | ||
| - | + | 2. The desired response is to increase Salicylic Acid levels when a pest gets near the bacteria. With the original system, salicylic acid increased but not as much as required. Thus, we tried putting the promoter activated by Lux next to the CI promoter in order to see if an increase was observed. With results of simulations, it was discovered that this solution optimized the increase of salicylic acid in the response. | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
<html> | <html> | ||
| Line 62: | Line 41: | ||
</html> | </html> | ||
| - | + | 3. Looking for the right set of parameters, it was possible conclude that Hill constant "k" (Concentration of the substrate when the rate of production is half of the maximum production rate) for the promoter activated by Lux '''had''' to be four times greater than the Hill constant for CI promoter. This results are based on biological reasons; the Lux promoter came from a quorum sensing system, then it needs high concentration of activator. Biologically, this is because it informs the promoter that there is high cell density. On the other hand, the CI promoter box came from a bacteriophage and it is used to attack the bacteria as quickly as possible, then it needs small quantities of protein to fully activate this system. | |
<html> | <html> | ||
| Line 69: | Line 48: | ||
</html> | </html> | ||
| - | + | The new system is showed in the figure below: | |
<html> | <html> | ||
| Line 76: | Line 55: | ||
</html> | </html> | ||
| - | + | [[File:modelocorr.jpg|thumb|500px|center|Figure 2. Detection module located in the plasmid I]] | |
<html> | <html> | ||
| Line 83: | Line 62: | ||
</html> | </html> | ||
| - | |||
| - | + | [[File:p2corr.png|250px|center|thumb|Figure 3. Alert system and toxin/antitoxin system located in the plasmid II ]] | |
| - | [[File: | + | |
</p> | </p> | ||
| Line 107: | Line 84: | ||
<br> | <br> | ||
| - | =='''Ralstonia:'''== | + | ===='''Ralstonia:'''==== |
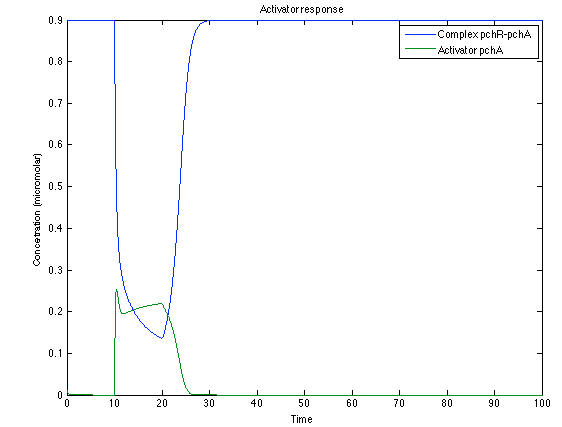
As it is shown below, when the system is under the presence of 3-OH-PAME the sensor is quickly phosphorylated and the complex phcR-phcA liberates the activator. This activator has a peak and then decrease softly because it is bound with the promoter. Once the impulse is gone, everything goes back to normality. | As it is shown below, when the system is under the presence of 3-OH-PAME the sensor is quickly phosphorylated and the complex phcR-phcA liberates the activator. This activator has a peak and then decrease softly because it is bound with the promoter. Once the impulse is gone, everything goes back to normality. | ||
| Line 116: | Line 93: | ||
</html> | </html> | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | [[File:Ral1.png|center|450x450pxpx]] [[File: ral2.png|center|450x450pxpx]] | + | [[File:Ral1.png|center|thumb|450x450pxpx|Figure 4. Behavior of the sensor phcS ]] |
| + | [[File: ral2.png|center|thumb|450x450pxpx|Figure 5. Behavior of the activator response ]] | ||
| Line 131: | Line 105: | ||
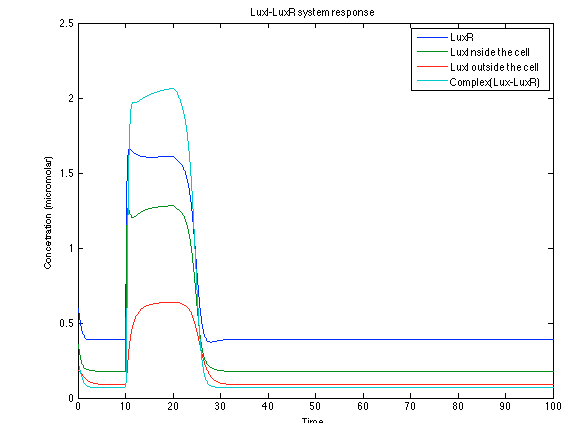
| - | [[File: ral3.png|center|450x450pxpx]] | + | [[File: ral3.png|center|thumb|450x450pxpx|Figure 6. Behavior of the LuxI - LuxR response ]] |
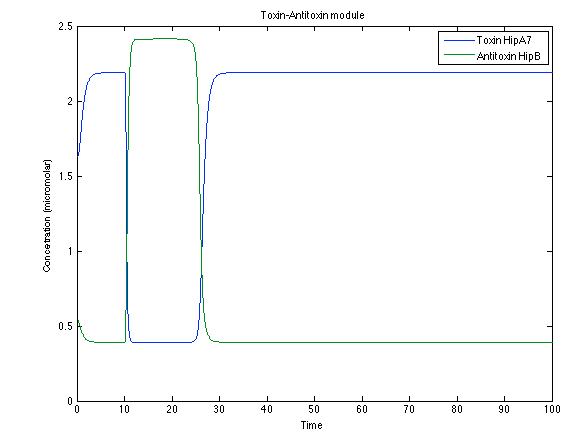
Looking the behavior of the species of interest, it possible to conclude that the antitoxin has greater concentration than the toxin when the 3-OH-PAME appears. This means that the cell is awake and can produce proteins. On the other hand, the Salicylic Acid has an increase of almost two times than observed before. | Looking the behavior of the species of interest, it possible to conclude that the antitoxin has greater concentration than the toxin when the 3-OH-PAME appears. This means that the cell is awake and can produce proteins. On the other hand, the Salicylic Acid has an increase of almost two times than observed before. | ||
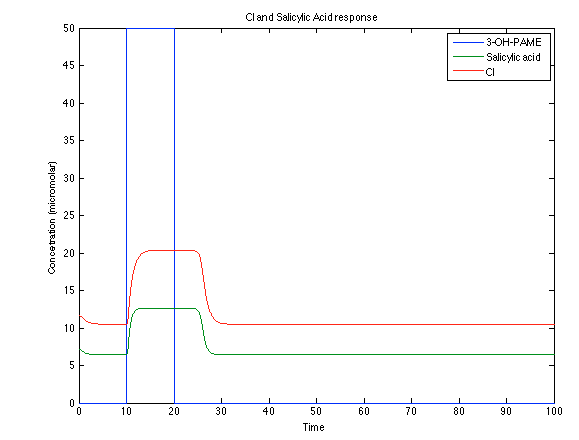
| - | [[File:Ral4.png|center|450x450pxpx]] [[File: ral5.png|center|450x450pxpx]] | + | [[File:Ral4.png|center|thumb|450x450pxpx|Figure 7. Toxin/Antitoxin response]] [[File: ral5.png|center|thumb|450x450pxpx|Figure 8. CI and Salycilic Acid response ]] |
<br> | <br> | ||
| - | + | ===='''Rust:'''==== | |
| - | =='''Rust:'''== | + | |
When the system is under the presence of chitin, it possible to see how the positive feedback of the chitoporin and chitinase works making their concentration increase. Consequently, it results in level increase of chitin monomers and release of the sensor which is going to activate the LuxI and LuxR promoter. | When the system is under the presence of chitin, it possible to see how the positive feedback of the chitoporin and chitinase works making their concentration increase. Consequently, it results in level increase of chitin monomers and release of the sensor which is going to activate the LuxI and LuxR promoter. | ||
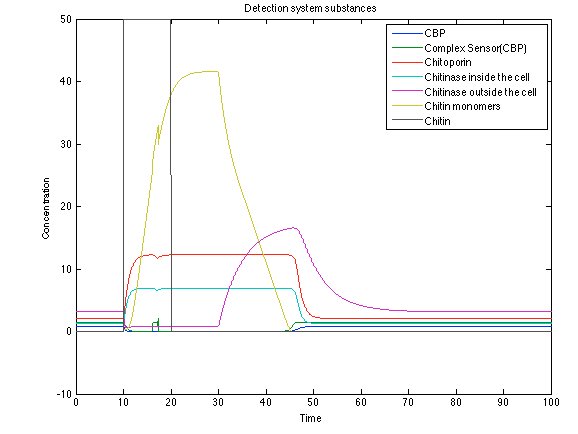
| - | [[File: rust1.png|center|450x450pxpx]] | + | [[File: rust1.png|center|450x450pxpx|thumb|Figure 9. Detection system substances]] |
Like it was shown in Ralstonia system, the Lux system concentrations increase when their promoter is activated. The complex LuxI-LuxR has a peak and then decrease because it is delay activation of CI and Salicylic Acid. | Like it was shown in Ralstonia system, the Lux system concentrations increase when their promoter is activated. The complex LuxI-LuxR has a peak and then decrease because it is delay activation of CI and Salicylic Acid. | ||
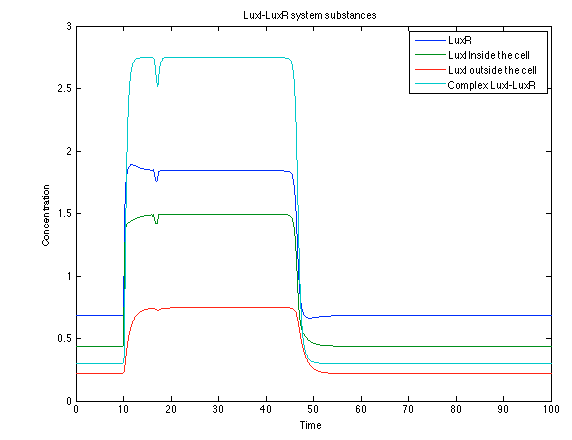
| - | [[File: rust2.png|center|450x450pxpx]] | + | [[File: rust2.png|center|thumb|450x450pxpx|Figure 10. Lux I - LuxR system substances]] |
The substances of interest behave just like expected. As in Ralstonia, the cell is awake when the chitin is present and the Salicylic Acid has folded its level of concentration. It is important to conclude that this system takes more time to go back to the steady state than Ralstonia's system. | The substances of interest behave just like expected. As in Ralstonia, the cell is awake when the chitin is present and the Salicylic Acid has folded its level of concentration. It is important to conclude that this system takes more time to go back to the steady state than Ralstonia's system. | ||
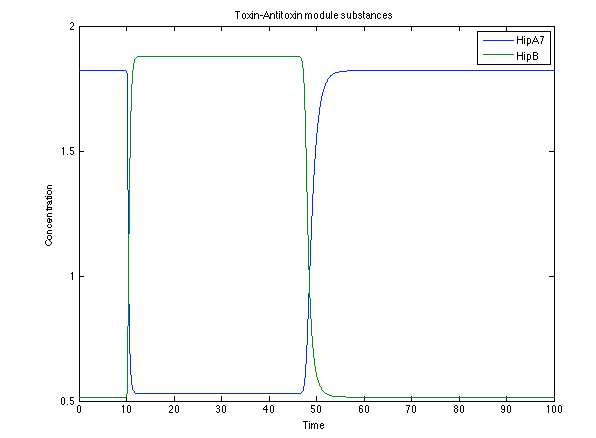
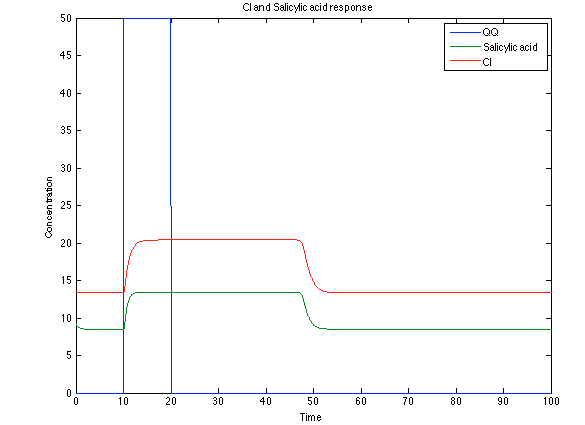
| - | [[File:Rust4.png|center|450x450pxpx]] [[File: rust5.png|center|450x450pxpx]] | + | [[File:Rust4.png|center|thumb|450x450pxpx|Figure 11. Toxin/Antitoxin module substances]] [[File: rust5.png|center|thumb|450x450pxpx|Figure 12. CI and Salycilic Acid response]] |
</p> | </p> | ||
</div> | </div> | ||
Latest revision as of 03:33, 27 October 2012
Template:Https://2012.igem.org/User:Tabima
Contents |
Results
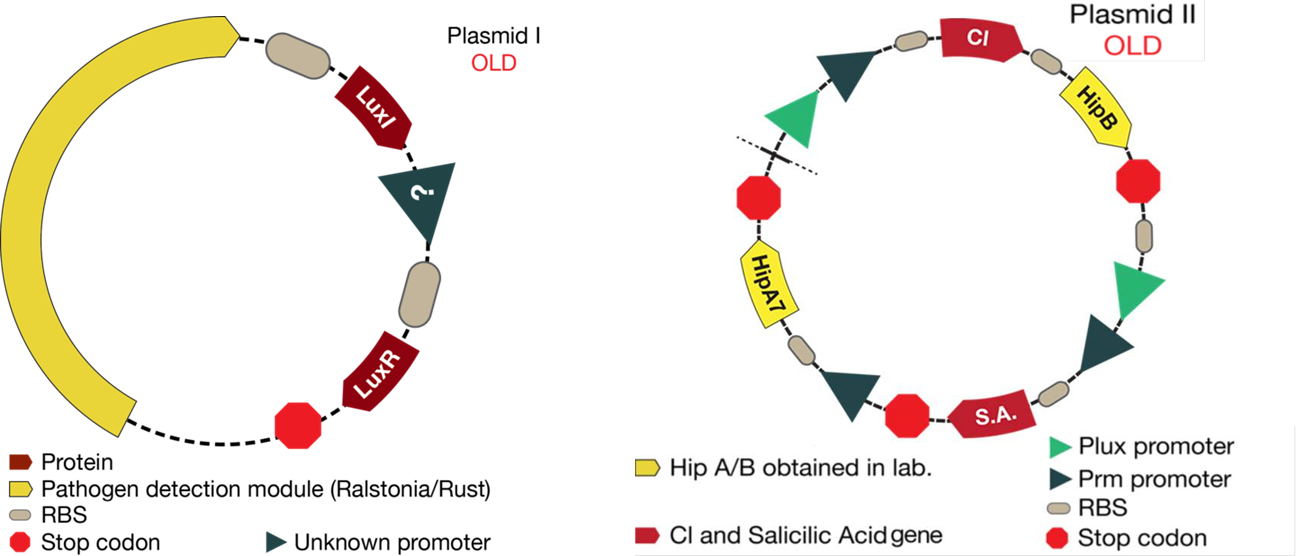
The mathematical model should help the experimental design to optimize the circuit. This case was not the exception. The figure below shows the original design of the circuit.
Once results from simulations were obtained ,we had to make little changes to the proposed system:
1. As it is shown, before results of simulations, there was an unknown promoter for LuxR. In the beginning, it was established that this promoter must had been constitutive. From simulations results we conclude that response curves of the system were not consistent with the reality (LuxR had giant concentrations and the salicylic acid never went up). In the designed system, LuxR had to interact with LuxI and turn on the response. Thus, it was thought that LuxR must had been in a similar concentration of LuxI. Consequently, LuxI was putted under the same promoter of LuxR, hence the two proteins will be promoted at the same time.
2. The desired response is to increase Salicylic Acid levels when a pest gets near the bacteria. With the original system, salicylic acid increased but not as much as required. Thus, we tried putting the promoter activated by Lux next to the CI promoter in order to see if an increase was observed. With results of simulations, it was discovered that this solution optimized the increase of salicylic acid in the response.
3. Looking for the right set of parameters, it was possible conclude that Hill constant "k" (Concentration of the substrate when the rate of production is half of the maximum production rate) for the promoter activated by Lux had to be four times greater than the Hill constant for CI promoter. This results are based on biological reasons; the Lux promoter came from a quorum sensing system, then it needs high concentration of activator. Biologically, this is because it informs the promoter that there is high cell density. On the other hand, the CI promoter box came from a bacteriophage and it is used to attack the bacteria as quickly as possible, then it needs small quantities of protein to fully activate this system.
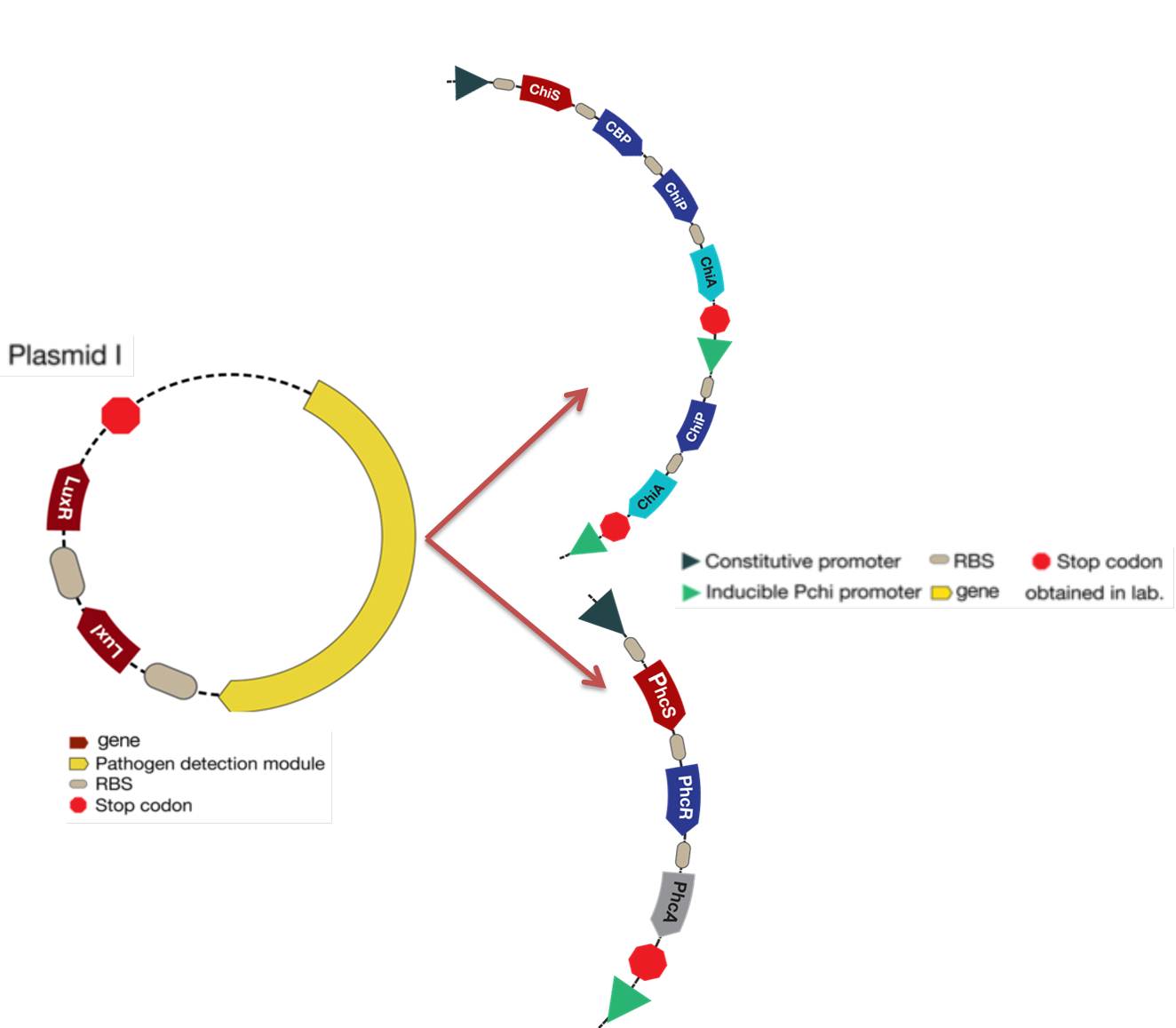
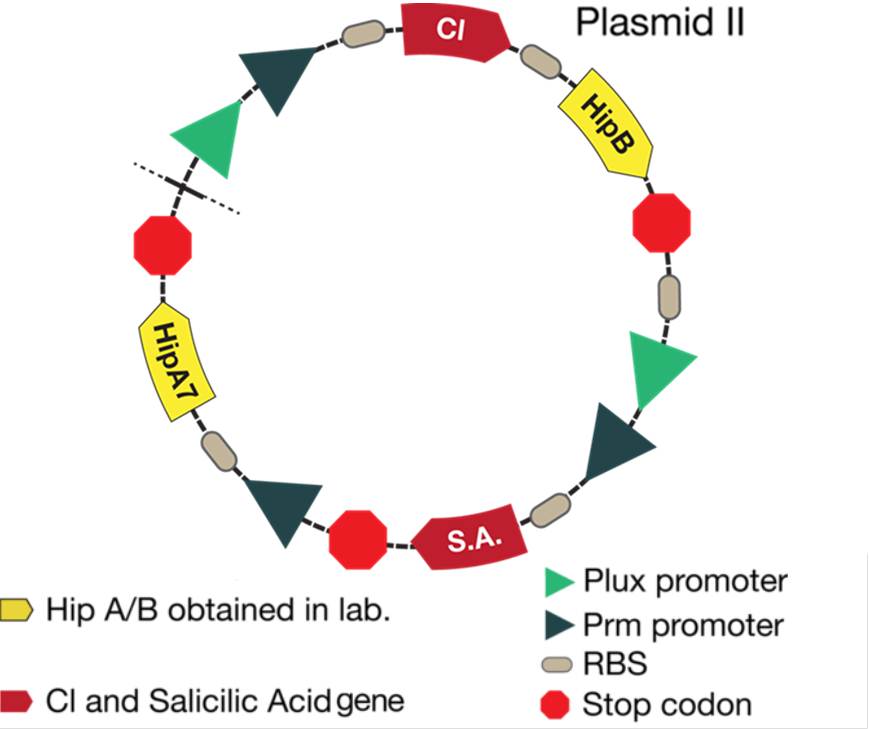
The new system is showed in the figure below:
Differential equations results
Although the screening of the parameters could not be completely done, we made a manual search for them and found a set that makes the system behave as expected. Here we present the mean response of all the substances in our biological system for one cell. The impulse of the pest was made during the times 10-20.
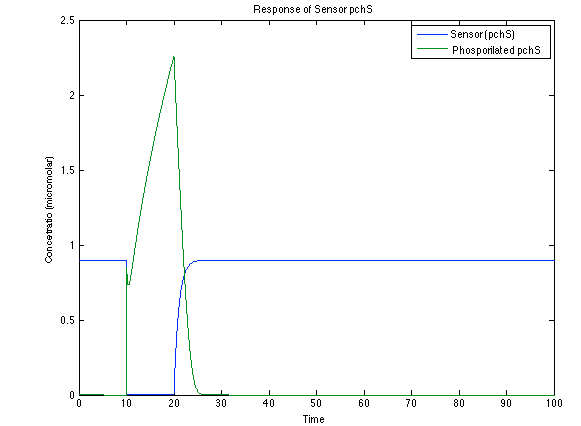
Ralstonia:
As it is shown below, when the system is under the presence of 3-OH-PAME the sensor is quickly phosphorylated and the complex phcR-phcA liberates the activator. This activator has a peak and then decrease softly because it is bound with the promoter. Once the impulse is gone, everything goes back to normality.
The LuxI- LuxR system increases its activations by phcsA
Looking the behavior of the species of interest, it possible to conclude that the antitoxin has greater concentration than the toxin when the 3-OH-PAME appears. This means that the cell is awake and can produce proteins. On the other hand, the Salicylic Acid has an increase of almost two times than observed before.
Rust:
When the system is under the presence of chitin, it possible to see how the positive feedback of the chitoporin and chitinase works making their concentration increase. Consequently, it results in level increase of chitin monomers and release of the sensor which is going to activate the LuxI and LuxR promoter.
Like it was shown in Ralstonia system, the Lux system concentrations increase when their promoter is activated. The complex LuxI-LuxR has a peak and then decrease because it is delay activation of CI and Salicylic Acid.
The substances of interest behave just like expected. As in Ralstonia, the cell is awake when the chitin is present and the Salicylic Acid has folded its level of concentration. It is important to conclude that this system takes more time to go back to the steady state than Ralstonia's system.
 "
"