|
|
| (49 intermediate revisions not shown) |
| Line 1: |
Line 1: |
| | <!-- Include the next line at the beginning of every page --> | | <!-- Include the next line at the beginning of every page --> |
| | {{:Team:LMU-Munich/Templates/Page Header|File:Team-LMU_streaked_plate.resized.jpg|3}} | | {{:Team:LMU-Munich/Templates/Page Header|File:Team-LMU_streaked_plate.resized.jpg|3}} |
| - | [[File:GerminationSTOP_banner.jpg|620px|link=]] | + | [[File:GerminationSTOP_bannerii.jpg|620px|link=]] |
| | | | |
| | | | |
| - | [[File:GerminationSTOP.png|100px|right|link=Team:LMU-Munich/Germination_Stop]] | + | [[File:GerminationSTOP.png|100px|right|link=]] |
| | + | |
| | | | |
| | | | |
| | == '''Germination'''STOP == | | == '''Germination'''STOP == |
| | + | <br> |
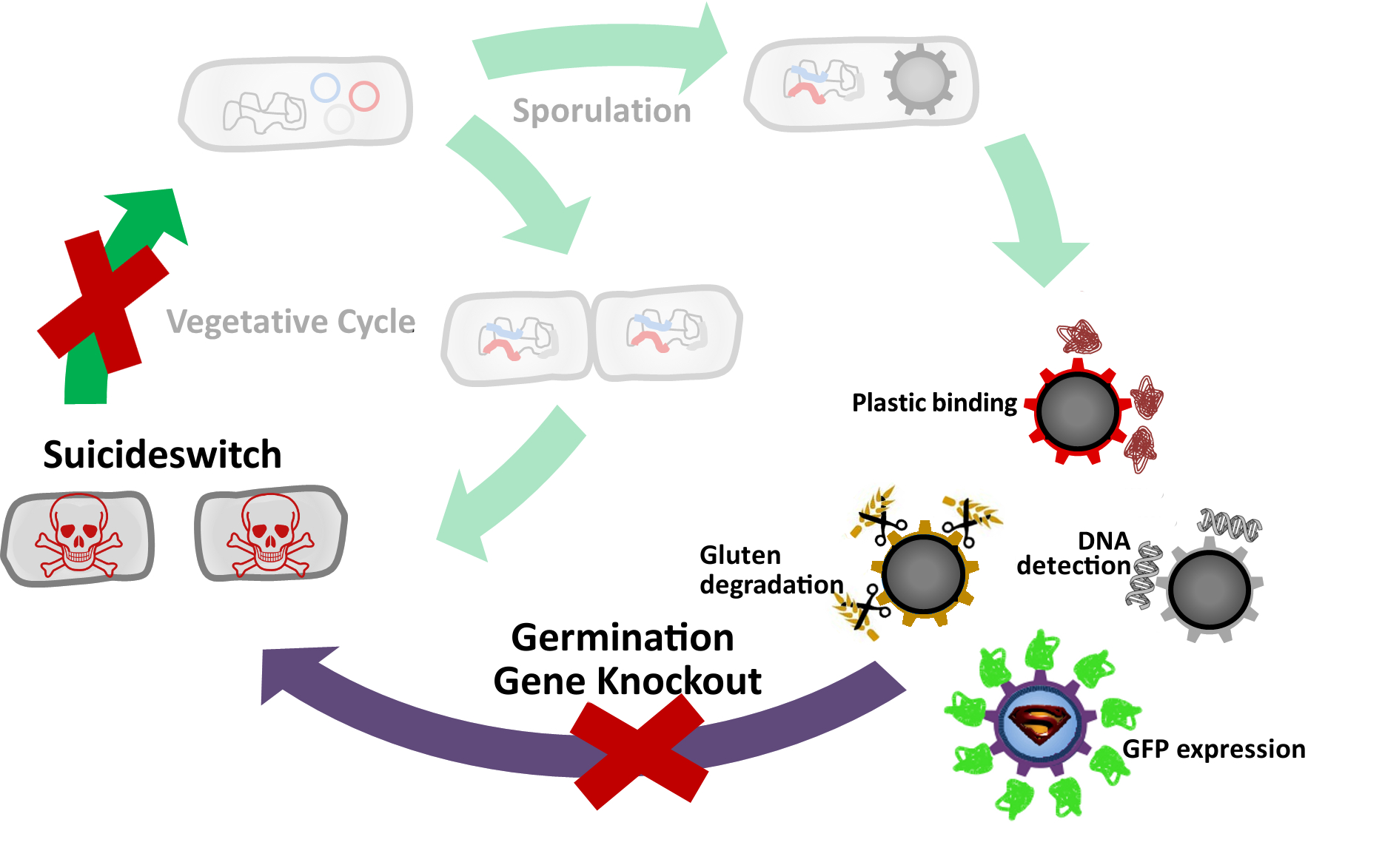
| | + | <p align="justify">The goal of this module was to yield [https://2012.igem.org/Team:LMU-Munich/Spore_Coat_Proteins '''Sporo'''beads] which are safe (unable to germinate) and consistently functional (maintain their spore shape and structure throughout time). To achieve this, we sought to remove the germination capability of our spores, while keeping their necessary structural functions intact.</p> |
| | | | |
| - | <p align="justify">The goal of this subproject was to yield [https://2012.igem.org/Team:LMU-Munich/Spore_Coat_Proteins '''Sporo'''beads] which are safe (unable to germinate) and consistently functional (maintain their spore shape and structure throughout time). To achieve this, we sought to remove the germination capability of our spores, while keeping their necessary structural functions intact.</p>
| |
| | | | |
| | + | [[File:GerminationSTOP_cycleIIii.jpg|620px|center]] |
| | | | |
| - | [[File:GerminationSTOP_cycleII.jpg|620px|center]] | + | [[File:NEXT.png|right|80px|link=Team:LMU-Munich/safetytour]] [[File:BACK.png|left|80px|link=Team:LMU-Munich/Bacillus_BioBricks]] |
| | | | |
| - | Two approaches were used to achieve this:
| |
| | | | |
| - | * [[Team:LMU-Munich/Germination_Stop#How do Germination Gene Knockouts Work?|Knock out]] genes that are involved in germination.
| |
| | | | |
| - | * [[Team:LMU-Munich/Germination_Stop#Suicide switch|'''Suicide''' switch]]: Toxin production by vegetative cells if germination knockout fails and spores manage to germinate.
| |
| | | | |
| | | | |
| - | ==How do Sporulation & Germination Work?==
| |
| | | | |
| - | {| style="color:black;" cellpadding="3" width="35%" cellspacing="15" border="0" align="right" style="text-align:left;"
| |
| - | | style="width: 70%;background-color: #EBFCE4;" |
| |
| - | {|
| |
| - | |[[File:sporulation_diagram.jpg|200px]]
| |
| - | |-
| |
| - | | style="width: 70%;background-color: #EBFCE4;" |
| |
| - | {| style="color:black;" cellpadding="0" width="85%" cellspacing="0" border="0" align="center" style="text-align:left;"
| |
| - | |style="width: 70%;background-color: #EBFCE4;" |
| |
| - | <font color="#000000"; size="2">Fig. 1: Taken from [http://www.ncbi.nlm.nih.gov/pubmed/19554258 Kim, J. & Schumann W (2009)]. <br />
| |
| - | '''A''': Cell at stage 0; vegetative phase. <br />
| |
| - | '''B''': Cell at stage II; asymmetric septum has been formed. <br />
| |
| - | '''C''': Cell at stage III; cytoplasmic membrane has engulfed forespore. <br />
| |
| - | '''D''': Cell at stage IV; coat formation has started; spore will be released from lysed mother cell.</font>
| |
| - | |}
| |
| - | |}
| |
| - | |}
| |
| | | | |
| - | <p align="justify">The ''Bacillus'' life cycle can include both classic division as well as reproduction by sporulation and spore germination.
| |
| - | In response to starvation of nutrients (including carbon, nitrogen, or phosphorus) or in response to peptides secreted by other cells which signal a high population densities, ''Bacillus'' cells form spores in a process called sporulation (Fig. 1).
| |
| - | The “mother” cell forms the endospore within its own cell membrane. The endospore contains its DNA in the spore core, which is protected by several layers of coats. The outermost layer is the spore crust. The spore is very dry, and contains a substance called dipicolinic acid (DPA), which is replaced with water when the spore germinates. Until the spore hydrates and swells out of its protective coats, it is resistant to a wide variety of environmental stressors, including UV radiation, toxic chemicals, freezing, high heat, dessication, and pH extremes. This resistance to stressors allows the spore to survive until conditions are good for growth.</p>
| |
| - | <p align="justify">On its inner spore membrane, the spore has germinant receptors. The spore coats are believed to be semipermeable or porous, in order to permit the passage of germinants to the receptors. When germinants such as amino acids and sugars reach the receptors, the spore begins the biochemical process of germination. It takes up water, shifts its pH, and swells. It breaks out of its coat and begins the outgrowth process (see Fig. 2). For our project, we wish to prevent the germination process.</p>
| |
| | | | |
| - | {| style="color:black;" cellpadding="3" width="70%" cellspacing="0" border="0" align="center" style="text-align:left;"
| |
| - | | style="width: 70%;background-color: #EBFCE4;" |
| |
| - | {|align:center
| |
| - | |[[File:germination_from_spore.jpg|620px|center]]
| |
| - | |-
| |
| - | | style="width: 80%;background-color: #EBFCE4;" |
| |
| - | {| style="color:black;" cellpadding="3" width="95%" cellspacing="0" border="0" align="left" style="text-align:left;"
| |
| - | |style="width: 70%;background-color: #EBFCE4;" |
| |
| - | <font color="#000000"; size="2">Fig. 2: '''The germination process in ''Bacillus'' spores.''' Taken from [http://www.ncbi.nlm.nih.gov/pubmed/14662349 Setlow 2003]. </font>
| |
| - | |}
| |
| - | |}
| |
| - | |}
| |
| | | | |
| | | | |
| - | ==How do Germination Gene Knockouts Work?==
| |
| | | | |
| - | <p align="justify">Based on the work of others, we chose to knock out genes ''cwlJ'', ''sleB'', ''cwlB'', ''gerD'', and ''cwlD''. Past works showed:</p> | + | <div class="box"> |
| - | *'''''cwlJ'' and ''sleB''''': both genes code for lytic enzymes which are active in the process of germination. When knocked out together, germination frequency was reduced by 5 orders of magnitude.
| + | ==Gene Knockouts== |
| - | *'''''gerD'' and ''cwlB''''': the ''gerD'' product plays unknown role in nutrient germination; ''cwlB'''s product plays role in cell wall turnover & cell lysis. When knocked out together, germination frequency was reduced by 5 orders of magnitude. Note: after several attempts to knock out ''cwlB'', and yielding cells unable to grow or cells growing at a very slow rate, we determined that knocking out ''cwlB'' would not be prudent for the production of [https://2012.igem.org/Team:LMU-Munich/Spore_Coat_Proteins '''Sporo'''beads], as it would delay all experiments in the '''Germination'''STOP module.
| + | {| "width=100%" style="text-align:center;" style="align:right"| |
| - | *'''''cwlD''''': this gene codes for recognition components for cleavage by the germination-specific cortex lytic enzymes. When knocked out, germination occurred at a rate of 0.003 to 0.05%.
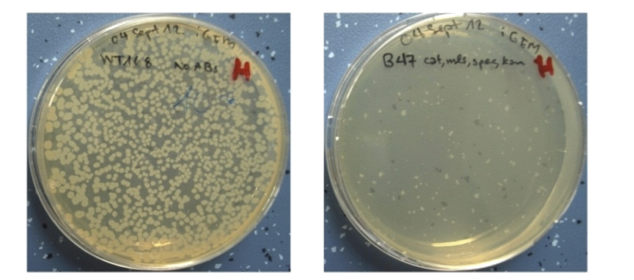
| + | |<p align="justify">We picked important germination genes and knocked them out. Subsequently we combined the single mutants into quadruple mutants and successfully prevented germination.</p> |
| - | <p align="justify">By knocking out all five of these genes, our goal was to yield a ''B. subtilis'' strain which produces spores completely incapable of germination. The stop to germination comes during the process of spore coat breakdown. Without the lytic enzymes to break down the coat, the spores should be unable to outgrow into the vegetative stage. | + | |[[File:LMU Germination STop plate.png|200px|right|link=Team:LMU-Munich/Germination_Stop/Knockout]] |
| - | After several attempts to knock out ''cwlB'', and producing extremely slow-growing mutants, ''cwlB'' was removed from our list of genes to knock out.</p>
| + | |
| - | | + | |
| - | {| class="colored"
| + | |
| - | !Germination Genes
| + | |
| - | !Gene Function
| + | |
| - | !Mutant Germination Rate
| + | |
| - | |- | + | |
| - | !''gerD''
| + | |
| - | |Unknown role in nutrition germination | + | |
| - | |Reduction by 5 orders of magnitude
| + | |
| - | |- | + | |
| - | !''cwlJ''
| + | |
| - | |Germination-specific lytic enzymes
| + | |
| - | |0.003 – 0.05%
| + | |
| - | No ATP detected
| + | |
| - | |-
| + | |
| - | !''sleB''
| + | |
| - | |Germination-specific lytic enzymes
| + | |
| - | |0.003 – 0.05%
| + | |
| - | No ATP detected
| + | |
| | |- | | |- |
| - | !''cwlD'' | + | ! colspan="2" |[[File:LMU Arrow purple.png|40px|link=Team:LMU-Munich/Germination_Stop/Knockout]] |
| - | |Recognition component for lytic enzymes | + | |
| - | |0.003 – 0-005%
| + | |
| | |} | | |} |
| | + | </div> |
| | | | |
| - | | + | <div class="box"> |
| - | | + | =='''Suicide'''switch== |
| - | ==What Methods Did We Use to Knockout Germination Genes?==
| + | {| "width=100%" style="text-align:center;" style="align:right"| |
| - | | + | |<p align="justify">In case of germination gene knockout failure, we invented the suicide switch. If spores still germinate, the production of a toxin leads to immediate cell death.</p> |
| - | <p align="justify">Two methods were employed to knock out germination genes: replacement by resistance cassettes and clean deletions. Resistance cassette (RC) knockouts were performed using long-flanking homology PCR (see Fig. 3 and [https://2012.igem.org/Team:LMU-Munich/Lab_Notebook/Protocols Protocols]). Single RC knockouts were created first; then they were combined to create multiple knockouts. A graphical representation of the genome with all replacements with resistance cassettes can be seen in Fig 4.</p> | + | |[[File:LMU SuicideSwitch grafik.png|200px|right|link=Team:LMU-Munich/Germination_Stop/SuicideSwitch]] |
| - | | + | |
| - | {| style="color:black;" cellpadding="3" width="70%" cellspacing="0" border="0" align="center" style="text-align:left;" | + | |
| - | | style="width: 70%;background-color: #EBFCE4;" |
| + | |
| - | {|align:center
| + | |
| - | |[[File:lfhPCR_ii.jpg|620px|center]] | + | |
| | |- | | |- |
| - | | style="width: 80%;background-color: #EBFCE4;" |
| + | ! colspan="2" |[[File:LMU Arrow purple.png|40px|link=Team:LMU-Munich/Germination_Stop/SuicideSwitch]] |
| - | {| style="color:black;" cellpadding="3" width="95%" cellspacing="0" border="0" align="left" style="text-align:left;"
| + | |
| - | |style="width: 70%;background-color: #EBFCE4;" | | + | |
| - | <font color="#000000"; size="2">Fig. 3: '''Procedure of long-flanking homology PCR.''' As an example, replacement of ''cwlD'' by a kanamycin (kan) resistance cassette is shown. 1000 base-pair fragments flanking ''cwlD'' as well as the kan cassette were amplified. The up-reverse and down-forward primers have overhangs complementary to the kan cassette. The up and down ''clwD'' fragments and the amplified kan cassette were fused in a PCR reaction. The result is a fragment containing the kan cassette flanked by the up- and downstream region ''cwlD''. ''B. subtilis'' is transformed with the fragment and the replacement of cwlD by the kan cassette is checked by PCR.</font>
| + | |
| | |} | | |} |
| - | |}
| + | </div> |
| - | |}
| + | <br> |
| - | | + | <br> |
| - | | + | <br> |
| - | {| style="color:black;" cellpadding="3" width="70%" cellspacing="0" border="0" align="center" style="text-align:left;"
| + | <br> |
| - | | style="width: 70%;background-color: #EBFCE4;" |
| + | |
| - | {|align:center
| + | |
| - | |[[File:germination_gene_knockouts_circle.jpg|620px|center]]
| + | |
| - | |-
| + | |
| - | | style="width: 80%;background-color: #EBFCE4;" |
| + | |
| - | {| style="color:black;" cellpadding="3" width="95%" cellspacing="0" border="0" align="left" style="text-align:left;"
| + | |
| - | |style="width: 70%;background-color: #EBFCE4;" |
| + | |
| - | <font color="#000000"; size="2">Fig. 4: Schematic representation of the B. subtilis chromosome with four germination genes replaced by different resistance cassettes. For more information about these knockout strains, please see our [https://2012.igem.org/Team:LMU-Munich/Strains Strain Collection].</font>
| + | |
| - | |}
| + | |
| - | |}
| + | |
| - | |}
| + | |
| - | | + | |
| - | | + | |
| - | <p align="justify">The germination rate of our mutants were checked with a [[Team:LMU-Munich/Lab_Notebook/Protocols|germination assay]]. The assay was developed based on the protocols of other researchers.
| + | |
| - | <br></p> | + | |
| - | <p align="justify">We were able to achieve germination rates as low as '''<1 in 4.6x10<sup>9</sup>''' spores for some mutants. A table of germination rates of different mutants and all other results can be found on [https://2012.igem.org/Team:LMU-Munich/Data/Knockout our results page].
| + | |
| - | <br></p> | + | |
| - | <p align="justify">Despite this success, safety was a top priority for this project. In the event that some small percentage of spores retained the ability to germinate, the '''Suicide''' switch subproject (below) prevents outgrowth into viable vegetative cells.</p>
| + | |
| - | | + | |
| - | | + | |
| - | =='''Suicide''' switch==
| + | |
| - | | + | |
| - | | + | |
| - | {| style="color:black;" cellpadding="3" width="70%" cellspacing="0" border="0" align="center" style="text-align:left;"
| + | |
| - | | style="width: 70%;background-color: #EBFCE4;" |
| + | |
| - | {|
| + | |
| - | |[[File:LMU SuicideSwitch grafik.png|624px|center]]
| + | |
| - | |-
| + | |
| - | | style="width: 70%;background-color: #EBFCE4;" |
| + | |
| - | {| style="color:black;" cellpadding="3" width="95%" cellspacing="0" border="0" align="left" style="text-align:left;"
| + | |
| - | |style="width: 70%;background-color: #EBFCE4;" |
| + | |
| - | <font color="#000000"; size="2">Fig. 5: Genetic elements of the '''Suicide''' switch and its expected performance.</font>
| + | |
| - | |}
| + | |
| - | |}
| + | |
| - | |}
| + | |
| - | | + | |
| - | | + | |
| - | <p align="justify">As a backup plan to make our <b>Sporo</b>beads even safer, we developed the <b>Suicide</b>switch. In case the spores do germinate, due to degradation or destruction of their outer coats, e.g. by high pressure, the <b>Suicide</b>switch will be turned on.<br></p>
| + | |
| - | <p align="justify">We take advantage of an alternative σ factor and a promoter regulated by it both not present in ''B. subtilis'' and therefore not recognised by any other σ factor of ''B. subtilis''.</p>
| + | |
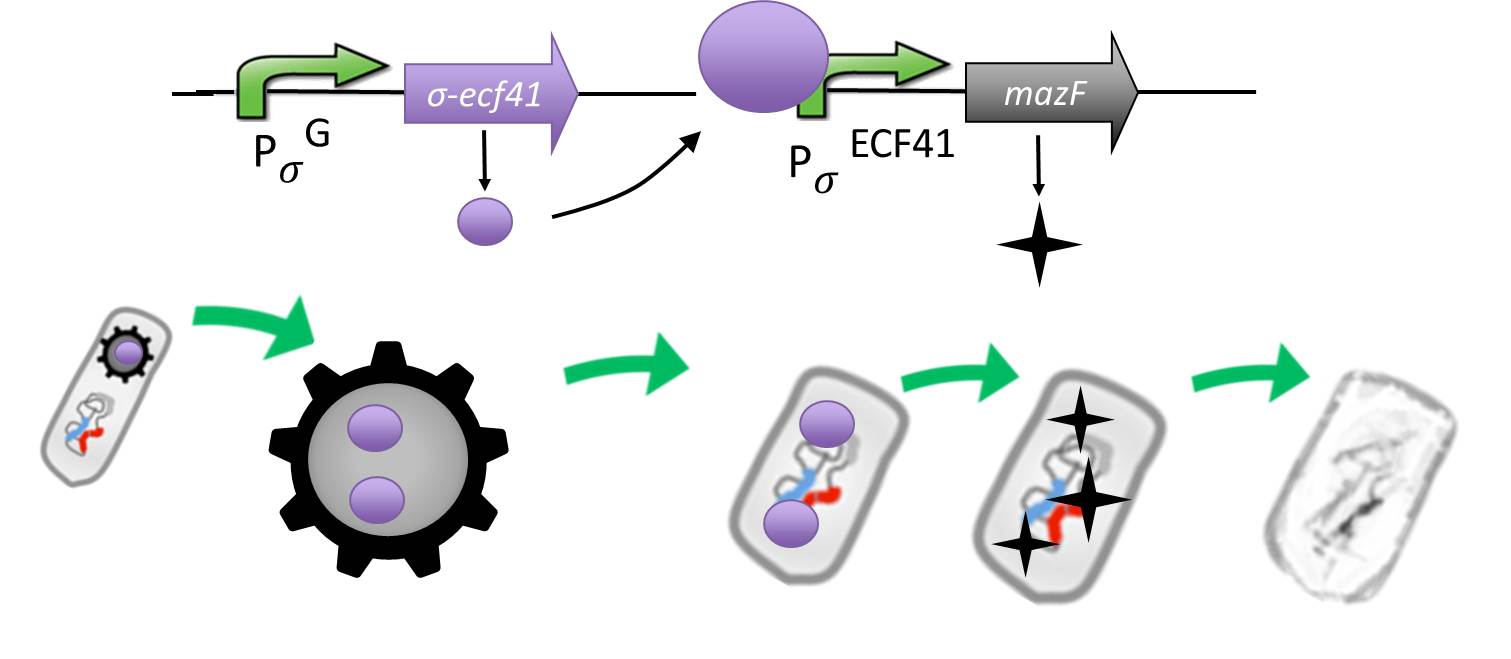
| - | <p align="justify">The alternative sigma factor [http://partsregistry.org/wiki/index.php?title=Part:BBa_K823043 ecf41<sub>Bli aa1-204</sub>] ([http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3426412/ Wecke T., Mascher T. 2012]), which derives from ''B. lincheniformes'' a truncated version constitutively on is synthetically linked to a sigma G regulated promotor responding quite late to σ<sup>G</sup> ([http://partsregistry.org/wiki/index.php?title=Part:BBa_K823048 P<sub>''spoIVB''</sub>] ([http://www.ncbi.nlm.nih.gov/pubmed/15699190 Steil L., Völker U. et al. 2005]) responding strongly or [http://partsregistry.org/wiki/index.php?title=Part:BBa_K823042 P<sub>sspK</sub>] ([http://www.ncbi.nlm.nih.gov/pubmed/15699190 Steil L., Völker U. et al. 2005]) responding weakly). σ<sup>G</sup> is the last σ factor activated in the forespore. Consequently ecf41<sub>Bli aa1-204</sub> is produced quite late in the forespore. ecf41<sub>Bli aa1-204</sub> then activates the [http://partsregistry.org/wiki/index.php?title=Part:BBa_K823041 P<sub>''ydfG''</sub>] ([http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3426412/ Wecke T., Mascher T. 2012]) promotor, which is the only target promoter of ecf41<sub>Bli aa1-204</sub>. This promoter is fused to [http://partsregistry.org/wiki/index.php?title=Part:BBa_K823044 MazF] ([http://jcs.biologists.org/content/118/19/4327.abstract Engelberg-Kulka H.,Amitai S. 2005]). Activation of P<sub>''ydfG''</sub> therefore leads to the expression of MazF, a bacterial toxin from ''E.coli'' degrading mRNA. <br></p>
| + | |
| - | <p align="justify">The timecourse is as follows: Sporulation of the <b>Sporo</b>beads leads to ecf41<sub>Bli aa1-204</sub>, through the activation of either P<sub>''spoIVB''</sub> or P<sub>sspK</sub>. This then would lead to the expression of MazF over the activation of P<sub>''ydfG''</sub>. This is either not toxic to the <b>Sporo</b>beads, as they do not rely on translation, or not produced any more by the <b>Sporo</b>beads as they are mature spores. After Germination MazF will then be produced for sure and kill the vegetative cell<br></p>
| + | |
| - | <p align="justify">
| + | |
| - | See what [https://2012.igem.org/Team:LMU-Munich/Data/Suicideswitch Data] we get when measuring a module with ''luxABCDE'' instead of MazF.
| + | |
| - | </p>
| + | |
| - | <p align="justify">
| + | |
| - | We are planning to model this system, but still need some data for it. We will get this data after finishing the cloning of P<sub>''sspK''</sub> and MazF into ''B. subtilis.</p>
| + | |
| - | | + | |
| - | | + | |
| - | | + | |
| - | | + | |
| - | | + | |
| - | | + | |
| - | | + | |
| | <div class="box"> | | <div class="box"> |
| | ====Project Navigation==== | | ====Project Navigation==== |







 "
"