Team:SJTU-BioX-Shanghai/Project/project2.2
From 2012.igem.org
(→Biosynthetic pathway) |
AleAlejandro (Talk | contribs) |
||
| (206 intermediate revisions not shown) | |||
| Line 24: | Line 24: | ||
<td valign="top" width="750"> | <td valign="top" width="750"> | ||
__NOTOC__ | __NOTOC__ | ||
| - | = | + | =Membrane Accelerator<br><br> - Fatty Acid and Alkane Biosynthesis= |
| + | {{Template:12SJTU_part_summary_head}} | ||
| + | *'''State of the art''' | ||
| + | Fatty acids are promising biofuel precursors with a broad and bright future. An enzyme system of nine proteins is responsible for fatty acid biosynthesis in ''E.coli'' and is first introduced to iGEM by our team. We choose four crucial enzymes, TesA, FabG, FabZ, and FabI, to prove the feasibility of the ''Membrane Accelerator'' and to enhance rate of fatty acids synthesis. Moreover, we tried to further accelerate the alkane biosynthetic pathway in ''E.coli''. | ||
| - | + | *'''Aims''' | |
| + | To identify and evaluate two outstanding features of the Membrane as a scaffold: the priority of products to exportation and the refinement of interaction. | ||
| - | + | To testify the feasibility of the ''Membrane Accelerator'' device in the context of fatty acids synthesis. | |
| - | + | To upgrade fatty-acid-producing ''Membrane Accelerator'' device with alkane synthesis function. | |
| - | + | To justify the universality of ''Membrane Accelerator'' | |
| - | + | *'''Achievements''' | |
| + | ''4 enzyme-bearing fusion proteins'' constructed that localize on membrane and assemble orderly. | ||
| - | + | ''50% yield increase'' of fatty acids sinply through localizing single TesA onto ''E.coli'''s inner membrane. | |
| + | ''24 fold of yield increase'' of fatty acids by localizing TesA, FabG, FabZ, and FabI onto ''E.coli'''s inner membrane. | ||
| - | + | ''fatty-acid-producing Membrane Accelerator upgrades'' by introducing additional two enzymes that convert fatty acids into alkane. | |
| - | + | ||
| - | + | {{Template:12SJTU_part_summary_foot}} | |
| - | |||
| - | == | + | ==Background== |
| - | + | We put ''Membrane Accelerator'' system into practice context to justify its versatility and efficiency. | |
| - | + | Fatty acid derivatives are to emerge as prominent fuels in the marketplace. Their high energy density and low water solubility make them promising alternatives to fossil fuel as transportation fuels. Biosynthesis, among all the biofuels production methods, is the most sustainable, environmental friendly and less controversial one. | |
| + | Initial fatty acid biosynthesis in ''E.coli'' is catalyzed by a series of nine enzymes and the final release of free fatty acids is catalyzed by a thioesterase via hydrolysis of acyl-ACP species. We are the first group in iGEM competition to recruit this biosynthetic pathway. ''Membrane Accelerator'' Device is expected to facilitate the biosynthesis of fatty acid in ''E.coli''. | ||
| - | + | Furthermore, we tried to upgrade fatty-acid-producing ''Membrane Accelerator'' by introducing additional two enzymes that convert fatty acids into alkane. Thus, alkane would be efficiently synthesized in ''E.coli'' directly. | |
| - | + | ===Biosynthetic Pathway=== | |
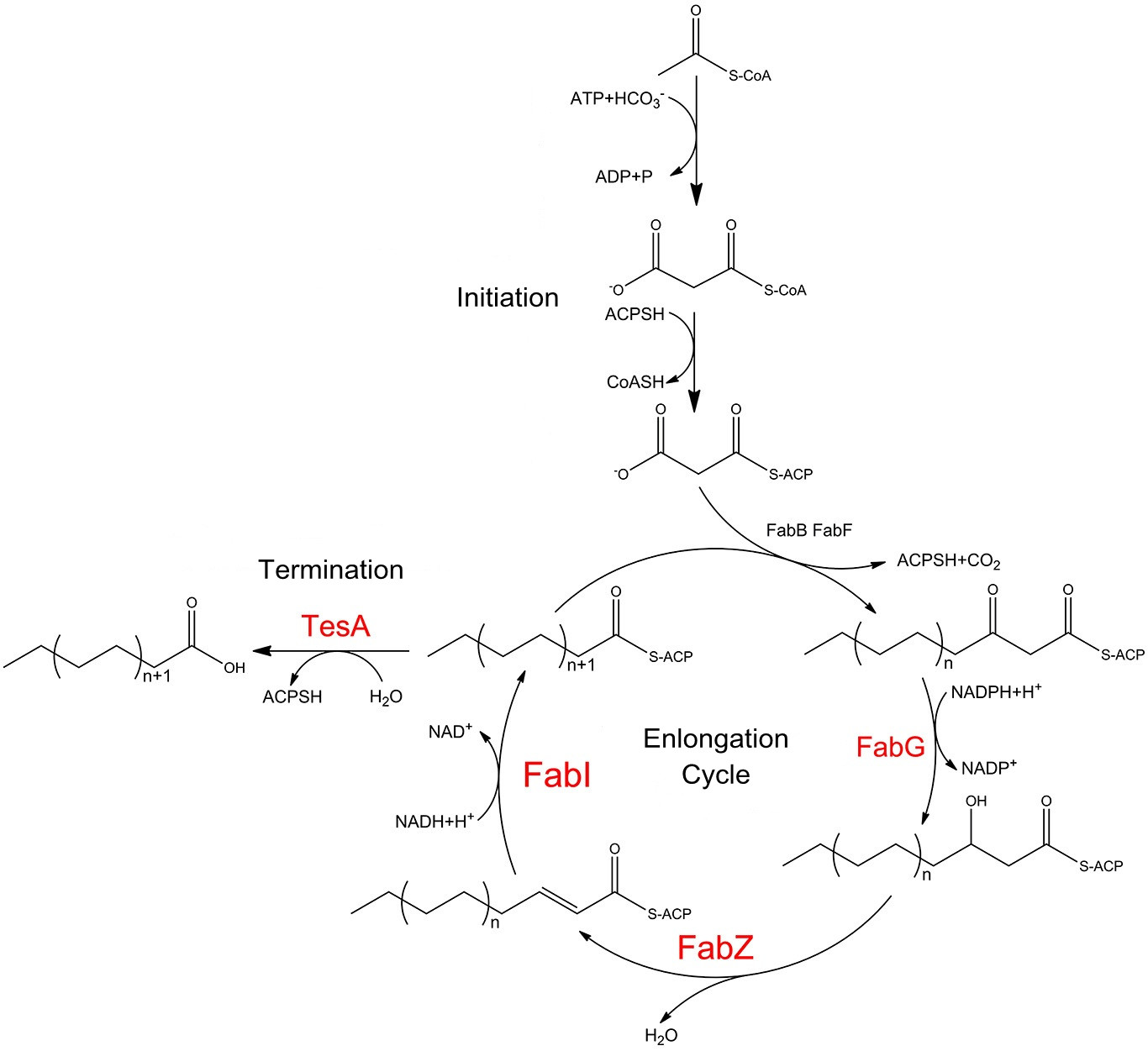
| - | '' | + | '''Fatty acid biosynthetic pathway''' |
| - | '' | + | Carbon chain elongation of fatty acid in ''E.coli'' is carried out by a nine-component enzyme system , FabA, FabB, FabD, FabF, FabG, FabH, FabI, FabZ, and ACP. They orderly cooperate to convert one equivalent of acetyl-CoA and 6-8 equivalents of malonyl-CoA into C14-C18 acyl-ACP species. The cytoplasmic mutant of the periplasmic thioesterase is capable of releasing free fatty acids preventing the fatty acyl yields from being directly harnessed for phospholipid biosynthesis. |
| - | |||
| - | + | [[File:12SJTU Fatty acid Biosynthetic Pathway s.jpg|thumb|center|650px|''Fig.1'' shows fatty acid biosynthesis pathways in ''E.coli'', stressing the role of FabG, FabZ, FabI and TesA.]] | |
| + | Catalytic cycle of the E. coli fatty acid synthesis is initiated when holo-ACP, NADPH and NADH, acetyl-CoA and malonyl-CoA undergo condensation and subsequent reduction to form butyryl-ACP. These reactions are catalyzed by the malonyl-CoA:ACP transacylase FabD, the ketosynthase FabH, the NADPH-dependent ketoreductase FabG, either the dual-function dehydratase/isomerase FabA or the monofunctional dehydratase FabZ, and the NADH-dependent enoyl reductase FabI. Then the butyryl-ACP is extended via 5-7 rounds of analogous reactions to produce a C14 to C18-ACP either fully saturated or monounsaturated. These extension cycles are catalyzed by either the ketosynthase FabB or FabF in collaboration with FabD, FabG, FabZ, and FabI. Finally, the full-length fatty acid is released from the corresponding fatty acyl-ACP via hydrolysis by C16-specific thioesterase,TesA. | ||
| - | + | FabG, FabI, FabZ play significant roles in the elongation of carbon chain of fatty acyl-ACP. It is TesA that processes fatty acyl-ACP into valuable products. Therefore, we recruited these four enzymes which together would finally make it possible to efficiently produce fatty acids with longer carbon chains which are much more preferable biofuel precursors. | |
| - | + | We have standardized the fatty acid biosynthesis related genes, TesA, FabG, FabI, FabZ, into BioBrick Parts [http://partsregistry.org/Part:BBa_K771301 Part:BBa_K771301], [http://partsregistry.org/Part:BBa_K771302 Part:BBa_K771302], [http://partsregistry.org/Part:BBa_K771303 Part:BBa_K771303], [http://partsregistry.org/Part:BBa_K771304 Part:BBa_K771304], [http://partsregistry.org/Part:BBa_K771305 Part:BBa_K771305], respectively. | |
| - | + | '''Alkane biosynthetic pathway''' | |
| - | : | + | 2011 iGEM team of [https://2011.igem.org/Team:Washington/Alkanes/Background Washington University] has introduced two enzymes that could convert ACP-Fatty Acid into alkane. The first enzyme is Acyl-ACP Reductase (AAR) which reduces long chain length acyl-ACPs into the corresponding fatty aldehydes. Long chain fatty aldehyde acts as a substrate for Aldehyde Decarbonylase (ADC), the second enzyme. The enzyme removes the carbonyl group (C=O) from the fatty aldehyde, yielding an alkane molecule one carbon shorter than the original Acyl-ACP and a molecule of formate. Note that FabA, FabB, FabD, FabF, FabG, FabH, FabI, FabZ, and ACP in ''E.coli could'' together synthesize long chain fatty acyl ACP that could be readily processed by AAR and ADC to produce alkane. |
| - | + | [[File:12SJTU Alkane Biosynthetic Pathway.png|thumb|center|600px|''Fig.2'' shows alkane synthetic pathway that originates from ''cyanobacteria'' species. Alkane Biosynthetic pathway is combined with Fatty Acid carbon-chain elongation pathway in ''E.coli'']] | |
| - | : | + | |
| - | + | ||
| - | === | + | ==Design of Experiment== |
| - | + | ||
| + | ===Membrane Accelerator in Fatty Acid Biosynthesis=== | ||
| + | Mechanism beneath our idea suggests the Membrane Scaffold enjoys two fundamental privileges: the Priority to Exportation and the Refinement of Interaction. We tested them in the context of fatty acid biosynthesis. | ||
| + | |||
| + | '''The Priority to Exportation''' | ||
| + | |||
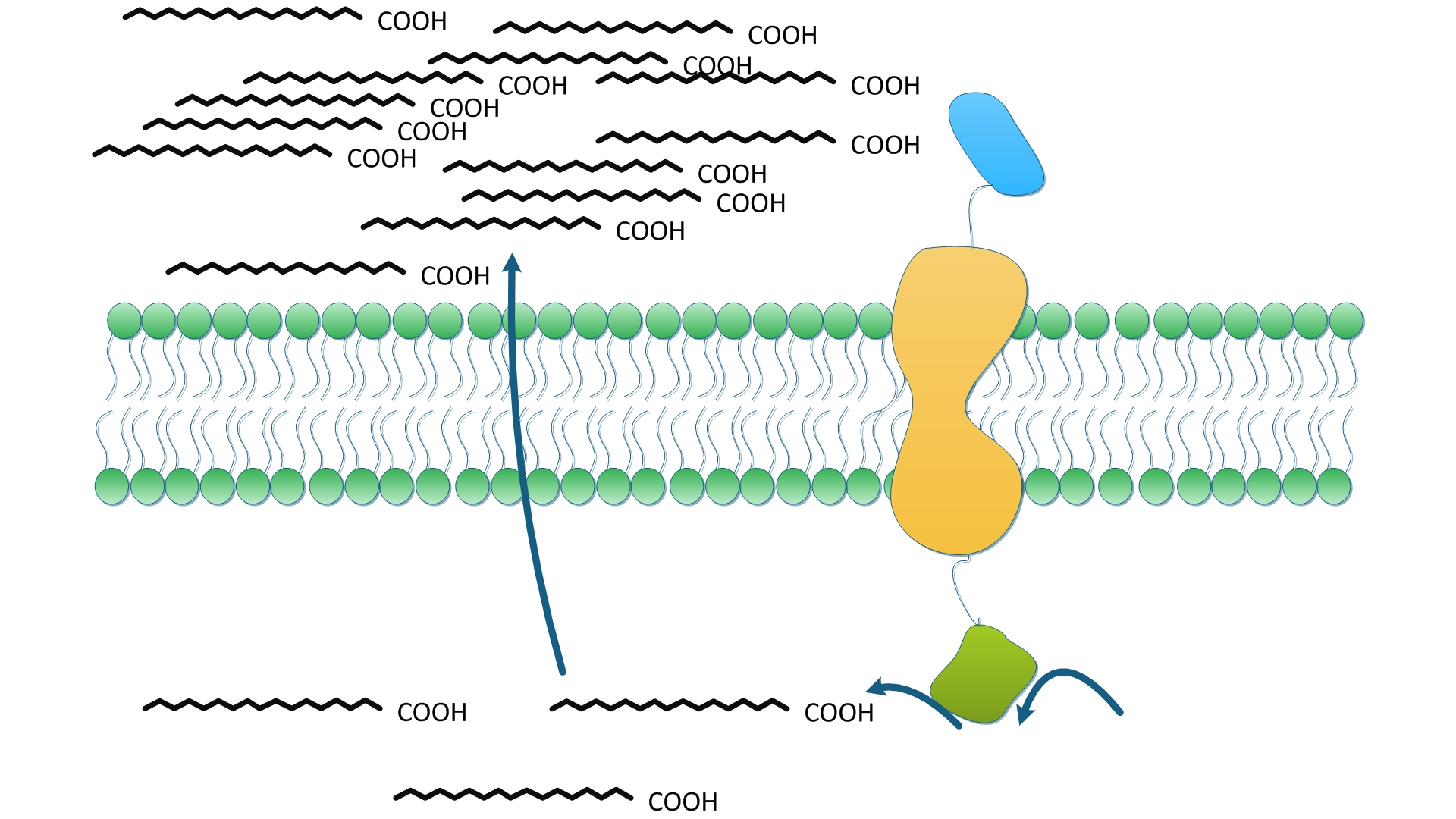
| + | [[File:12SJTU Fatty acid-wy-1.jpg|350px|right|thumb|''Fig.3'' shows how one solo enzyme anchored onto membrane could serve to facilitate the reaction in term of the priority to exportation.]] | ||
| + | The fatty acid synthesis is terminated by the hydrolysis of fatty acyl-ACP and the release of free fatty acids into cytoplasm. We suppose TesA anchored on membrane could effectively increase the concentration of fatty acids near membrane, which in turn, facilitates the transmembrane transportation of fatty acids. Higher level of fatty acids in the culture media makes it easier to obtain and purify product and more suitable for industrialized production. On the other hand, TesA removes fatty acyl-ACP from the reaction and thus, according to Le Châtlier principle, shifts the chemical equilibrium to accelerate and to accumulate more fatty acids. | ||
| + | |||
| + | To identify and to evaluate the priority of products to exportation, controlled experiments were designed and conducted. Wild type ''E.coli'' and ''E.coli'' overexpressing diffusing cytoplasmic TesA were used as control groups. TesA fused with Lgt was expressed in experimental group. | ||
| + | |||
| + | [[File:12SJTU membraneTesA.jpg|200px|center|thumb|''Fig.4'' shows the construction details of membrane anchored TestA]] | ||
| + | |||
| + | '''The Refinement of Interaction''' | ||
| + | |||
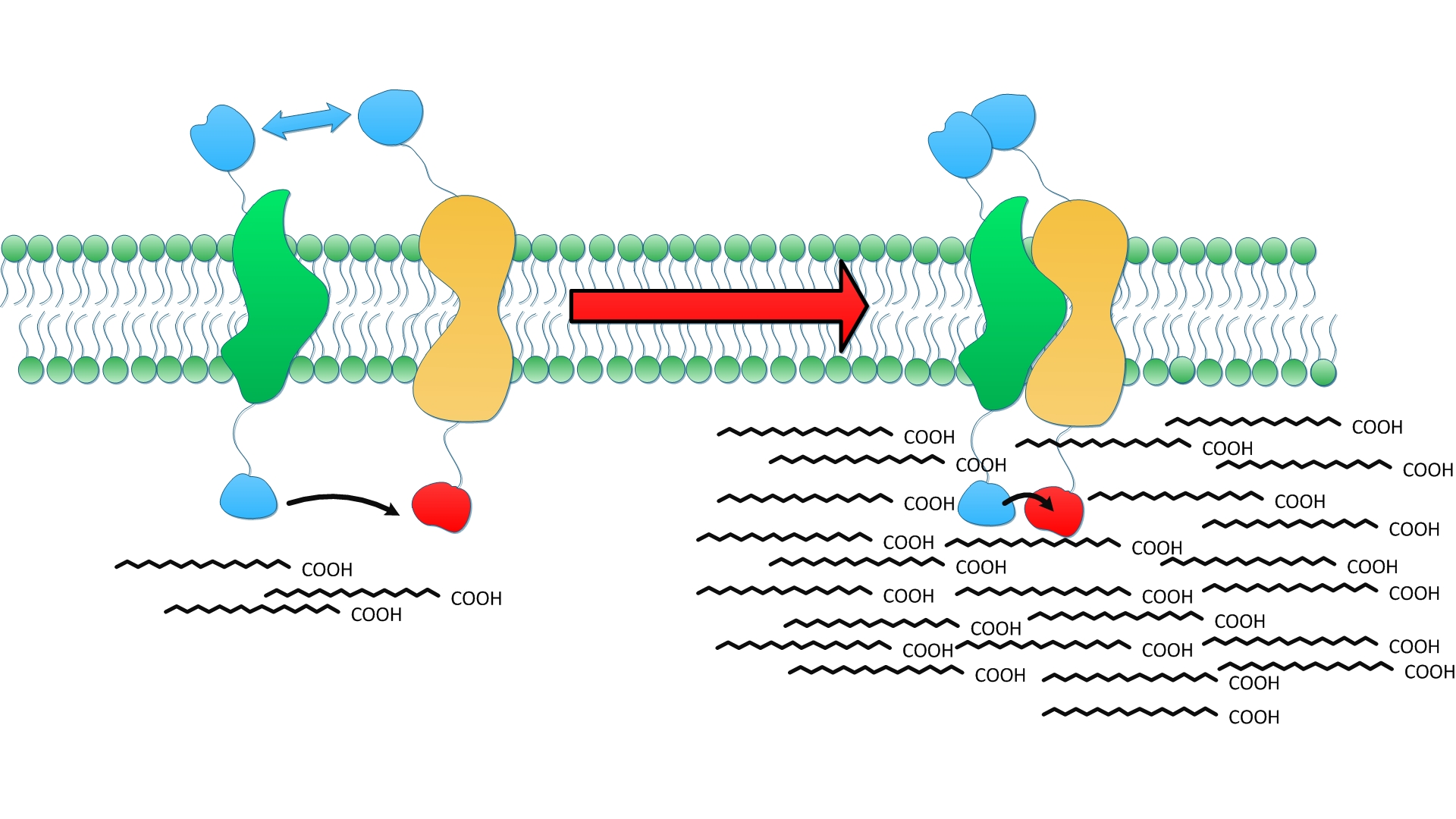
| + | [[File:12SJTU Fatty acid-wy-2.jpg|350px|right|thumb|''Fig.5'' shows how membrane could serve to refine and stabilize the interaction upon which enzymes aggregate and cooperate.]] | ||
| + | Enzymes fused with membrane anchors will be directed to the membrane as expected right after their translation. Thus, the distribution of enzymes is restricted to the 2D membrane rather than diffusing randomly throughout the cytoplasm. Due to spatial restriction, each membrane anchor is more easily to interact with each other. Moreover, the interaction could be stabilized by phospholipid around transmembrane domain. We believe a tandem of enzymes involved in sequential reactions could be organized on the membrane swiftly and orderly in ''Membrane Accelerator''. | ||
| + | |||
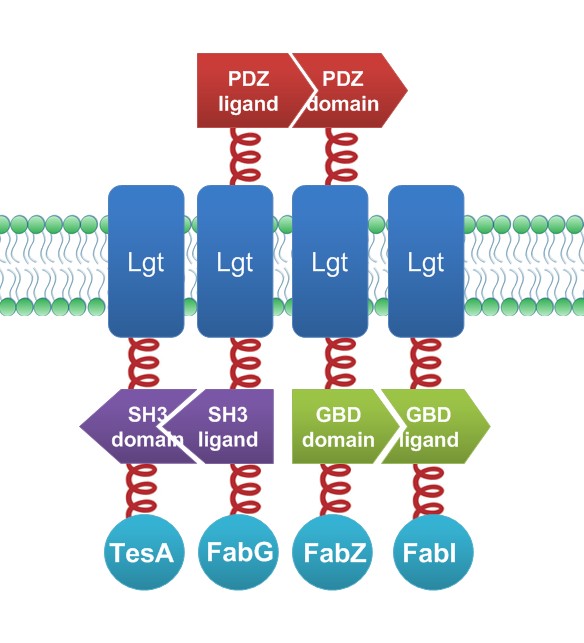
| + | To testify and to assess the refinement of interaction, controlled experiments were designed and conducted. Four enzymes are selected based on previous study. We express thioesterase(TesA) and full complement of reductive enzymes(FabG, FabZ and FabI) because previous studies showed moderate overexpression of TesA in ''E.coli'' gave rise to elevated fatty acid productivity and overexpression of reductive enzymes could lead to 50% increase in fatty acid turnover. Wild type ''E.coli'' and ''E.coli'' overexpressing diffusing TesA, FabG, FabZ and FabI were used as control groups. In experimental group, TesA, FabG, FabZ and FabI were linked to orderly organized Membrane Anchors, aligning with corresponding reactions occurring in sequence. All four fusion Membrane Anchors were co-expressed in one cell. | ||
| + | |||
| + | [[File:12SJTU Fatty acid accelerator.jpg|450px|center|thumb|''Fig.6'' demonstrates the construction details of ''Membrane Accelerator'' for use of fatty acid synthesis.]] | ||
| + | |||
| + | The Parts Registered as Membrane Anchor 2 without MS2-TesA, Membrane Anchor 3-FabG, Membrane Anchor 4-FabZ, Membrane Anchor 5 without VVD-FabI are [http://partsregistry.org/Part:BBa_K771305 Part:BBa_K771305], [http://partsregistry.org/Part:BBa_K771306 Part:BBa_K771306], [http://partsregistry.org/Part:BBa_K771307 Part:BBa_K771307], [http://partsregistry.org/Part:BBa_K771308 Part:BBa_K771308], respectively. | ||
| + | |||
| + | ===Membrane Accelerator in Alkane Biosynthesis=== | ||
| + | |||
| + | '''Flexibility of Reaction Space''' | ||
| + | |||
| + | [[File:12SJTU alkanereactionspace-1.jpg|350px|right|thumb|''Fig.7'' shows we can easily change the reaction space from cytoplasm to periplasm with ''Membrane Accelerator''.]] | ||
| + | |||
| + | As mentioned above, fatty acyl-ACP is produced in ''E.coli'' by a nine-component enzyme system, in which FabG, FabZ, FabI play crucial part. AAR and ADC could then convert fatty acyl-ACP into alkane, the main constituents of diesel fuel. At first, we planned to link FabG, FabZ, FabI, AAR and ADC with constitutively assembling membrane anchors to create a new ''Membrane Accelerator''. In this case, all enzymes face cytoplasm. However, a problem would occur because alkane is very hard to be exported to periplasm. To much alkane would pose stress on ''E.coli''. | ||
| + | |||
| + | As we know, aldehyde is more easily to be exported through cell membrane than alkane. Considering the uniqueness and adaptability of Membrane Scaffold system, we decided to place ADC in the periplasmic function domain of Membrane Anchor, as shown in ''Fig.7''. Thus, after the production of fatty aldehyde by AAR in cytoplasm near inner membrane, the long chain aldehyde molecule is readily exported to periplasm. In periplasm, fatty aldehyde is processed by ADC to be converted into free alkane. The upgraded ''Membrane Accelerator'' is expected to facilitate alkane production in ''E.coli'' not only by aggregating enzymes but also by improving alkane exportation. | ||
| + | |||
| + | We have constructed corresponding fusion proteins shown in ''Fig.8''. Full result and data analysis will be out soon. | ||
| + | |||
| + | [[File:12SJTU Alkane accelerator.jpg|450px|center|thumb|''Fig.8'' demonstrates the construction details of ''Membrane Accelerator'' for use of alkane synthesis.]] | ||
| + | |||
| + | ==Result and Discussion== | ||
| + | ===Membrane Accelerator in Fatty Acid Biosynthesis=== | ||
| + | |||
| + | '''Overview''' | ||
| + | |||
| + | Excitingly, the fatty acid biosynthesis was accelerated sharply by recruiting ''membrane accelerator''. The introduction of TesA on membrane alone results in '''50% increase in fatty acids yield'''. By gathering FabG, FabI, FabZ and TesA on the membrane, the production of fatty acid was '''enhanced significantly by 9 fold '''compared with ''E.coli'' overexpressing the same amount of corresponding cytoplasmic enzymes. The statistical data strongly support the superiority of ''Membrane Accelerator''. | ||
| + | |||
| + | To demonstrate the priority to exportation and the refinement of interaction, two sets of controlled experiments have been set as following: | ||
| + | |||
| + | '''''WT''''' stands for ''E.coli'' transformed with corresponding plasmid(s) without exogenous gene. | ||
| + | |||
| + | '''''F-''''' stands for ''E.coli'' overexpressing corresponding free cytoplasmic enzyme(s). F-TesA means free TesA. F-AGZI means free TesA, FabG, FabZ and FabI. | ||
| + | |||
| + | '''''M-''''' stands for ''E.coli'' expressing corresponding enzyme(s) linked to membrane anchor(s). M-TesA means membrane anchored TesA. M-AGZI means aggregating membrane anchored TesA, FabG, FabZ and FabI. | ||
| + | |||
| + | Statistical data are shown as below and GC-MS data are attached to https://2012.igem.org/Team:SJTU-BioX-Shanghai/Parts | ||
| + | |||
| + | |||
| + | '''Assay of the Priority to Exportation''' | ||
| + | |||
| + | The construction detail of experimental group is shown in ''Fig.4''. Control group is ''E.coli'' expressing the same amount of diffusing cytoplasmic TesA. | ||
| + | |||
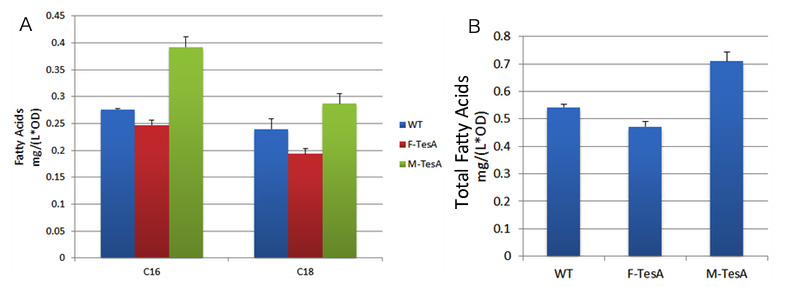
| + | TesA is responsible for the hydrolysis of fatty acyl-ACP, with special affinity to C16- and C18- precursor. As ''Fig.9 A'' indicates, 20 hours after induction, ''E.coli'' with membrane anchored TesA experienced a 50% increase in C-16, C-18 fatty acids content and total fatty acids (''Fig.9 B'') in supernatant, compared with ''E.coli'' with free TesA. | ||
| + | |||
| + | [[File:12SJTU-TesA-supernatant.png|thumb|center|750px|''Fig.9'' shows fatty acid content in supernatant of three groups to evaluate the advantages of membrane anchored TesA. A indicates C16/C18 fatty acids content among three groups. B stands for the total amount of fatty acids among three groups.]] | ||
| + | |||
| + | Fatty acids yielded in supernatant went up to 0.71mg/(L·OD). The result supports that membrane anchored TesA could efficiently transfer fatty acyl-ACP into desirable fatty acids right beneath inner membrane, thus making it much easier for the final products to diffuse into periplasmic space. | ||
| + | |||
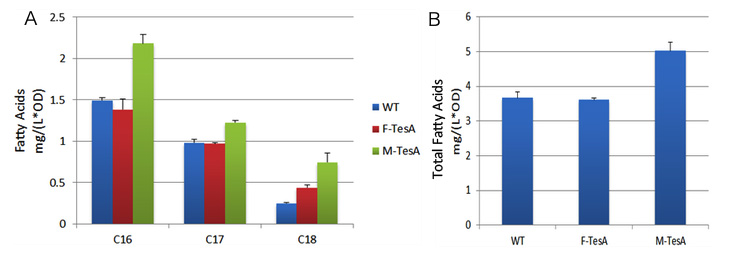
| + | [[File:12SJTU-TesA-sediment.png|thumb|center|750px|''Fig.10'' shows fatty acid content in the sediment of three groups to evaluate the advantages of membrane anchored TesA. A indicates C16/C18 fatty acids content among three groups. B stands for the total amount of fatty acids among three groups.]] | ||
| + | |||
| + | ''Fig.10'', on the other hand, showed fatty acids content in sedimentation also went up by 40%, up to 5.02mg/(L·OD). The result is still within our expectation since fatty acyl-ACP has been removed from the reaction system to form free fatty acids. As a result, the chemical equilibrium shifts and more fatty acids would be accumulated. | ||
| + | |||
| + | We also witnessed a slight decrease in ''E.coli expressing'' free TesA compared with the wildtype, which testifies the statement in a previous study that high levels of TesA inhibits fatty acids synthesis activity but could enhance the activity at low concentrations. | ||
| + | |||
| + | '''Assay of the Refinement of Interaction''' | ||
| + | |||
| + | We have tried to facilitate exportation of fatty acids by linking TesA to membrane protein. In this way, we accelerated the reaction and increased the turnover per unit time and finally make it possible to efficiently produce fatty acids with longer carbon chains which are much more preferable biofuel precursors. The result verifies one privilege of Membrane Scaffold: Priority to Exportation. Actually another privilege that Membrane Scaffold enjoys is that protein interaction is improved and stabilized due to the spatial restriction. Thus we could more easily assemble different enzymes together to decrease the distance that intermediates need to travel between each enzyme. In this way biochemical reaction should be accelerated sharply. | ||
| + | |||
| + | FabG, FabI, FabZ play significant roles in the elongation of carbon chain of fatty acyl-ACP. To optimize the productivity of the system we established, we tried to combine these two privileges (Priority to Exportation and Refinement of Interaction) together, gathering TesA, FabG, FabI and FabZ on membrane through protein interaction. Notable increase in both diversity and amount of fatty acids was detected compared to control group, which lends strong support to our hypothesis. | ||
| + | |||
| + | The construction detail of experimental group is shown in ''Fig.5''. Control group is ''E.coli'' expressing the same amount of diffusing cytoplasmic TesA, FabG, FabI and FabZ. | ||
| + | |||
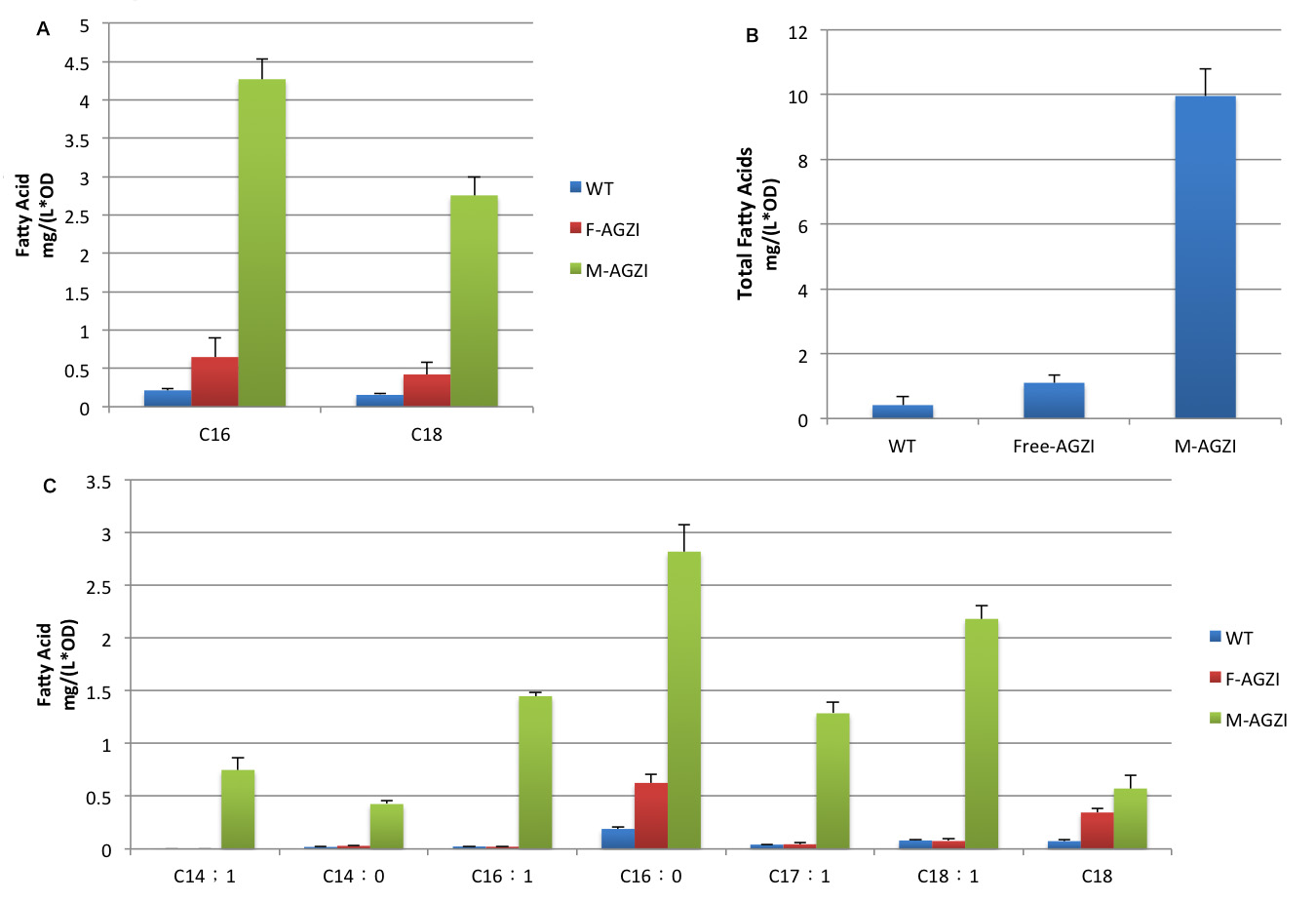
| + | [[File:12SJTU-fattyAGZI.png|thumb|center|750px|''Fig.11'' shows fatty acid content in supernatant of three groups to evaluate the advantages of membrane anchored TesA,FabG,FabZ and FabI. A indicates C16/C18 fatty acids content. B stands for the total amount of fatty acids. C shows the changes in products diversity.]] | ||
| + | |||
| + | Cluster of FabG, FabI and FabZ provides C16- and C18 specific TesA with sufficient amount of fatty acyl-ACP to hydrolyze and release. Therefore, we witnessed a tremendous growth in the turnover of fatty acids with C16 and C18 skeleton in the supernatant.(Fig.11 A) | ||
| + | |||
| + | Moreover, fatty acid with C14 skeleton was first detected in ''E.coli'' expressing membrane anchored enzymes compared with ones with free enzymes. It is probably because the high productivity of membrane anchored TesA quickly released free fatty acid. Monounsaturated fatty acids also emerge in considerable amount for the first time since TesA is located so closely to the cluster of FabG, FabI and FabZ that it catches intermediate with C16 and C18 skeleton even before they are reduced.(Fig.11 C) | ||
| + | |||
| + | Augment in both diversity and amount of fatty acids led to 24 fold increase in total fatty acid yield compared with wild type and 9 fold compared with ''E.coli'' expressing diffusing cytoplasmic enzymes. (Fig.11 B) The exciting result convincingly proves that membrane scaffold enhances protein interaction and cluster of enzymes makes reaction faster to a surprising extent. Products accumulated near inner membrane can traval a shorter distance to be exported to membrane. | ||
| + | |||
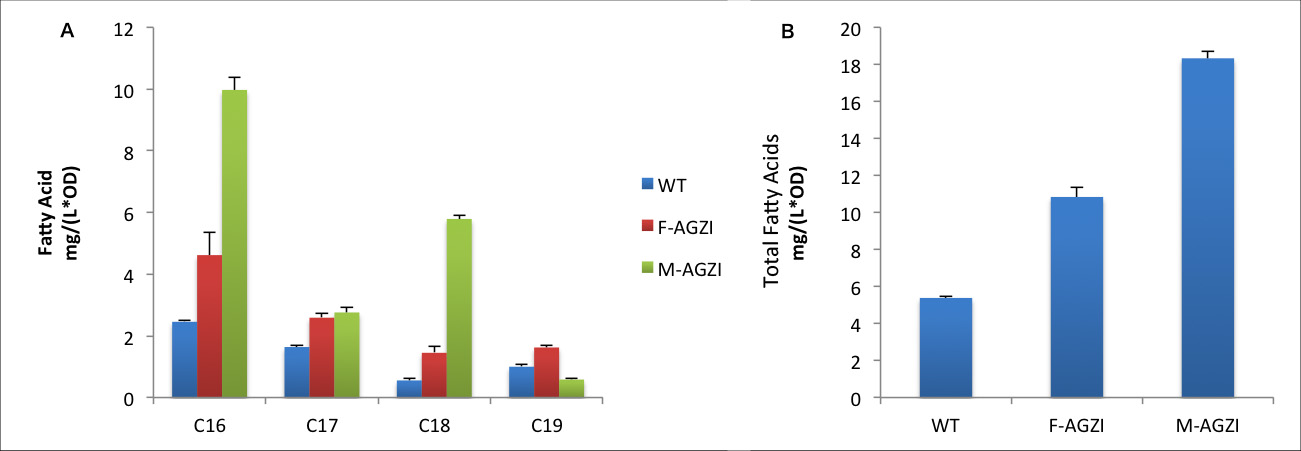
| + | [[File:12SJTU-123.png|thumb|center|750px|''Fig.12'' shows fatty acid content in sediment of three groups to evaluate the advantages of ''Membrane Accelerator''. A indicates C16/C18 fatty acids content. B stands for the total amount of fatty acids.]] | ||
| + | |||
| + | The fatty acids yields increase in sediment is relevantly moderate. The reason might be that membrane anchored enzymes facilitate exportation of large quantity of fatty acids so the amount of fatty acid remaining in cytoplasm is limited. It is notable that C19 fatty acids only exist in cytoplasm because large molecular weight of C19 fatty acid prevents it from exportation and finally they are trapped in the cell. C19 fatty acids yield in ''Membrane Accelerator'' Group is even less than Group with diffusing cytoplasmic enzymes. We suppose it is caused by the high efficiency of TesA which leads to quick release of free fatty acid. | ||
| + | |||
| + | ==Future Direction== | ||
| + | |||
| + | [[File:12SJTU_Cyano.jpg|350px|right|thumb|Fig.13 ''Cyanobacteria'' under light microscope]] | ||
| + | We haven't optimized experiment conditions with respect to strains, growth media, temperature etc., but the result has proved that ''Membrane Accelerator'' could accelerate biosynthetic pathway in ''E.coli'' marvelously. Moreover, changing the proportion of different enzymes in each enzyme assembly on membrane would promisingly enhance biosynthesis rate even more. In the near future, we expected to optimize those conditions to further improve the efficiency of ''Membrane Accelerator''. | ||
| + | |||
| + | To produce economic biofuel which could be put into mass production, we wanted to transform alkane-producing ''Membrane Accelerator'' into ''Cyanobacteria''. ''Cyanobacteria'' is an autotrophic species which could utilize carbon dioxide and light to produce varieties of organics. Someday we might engineered a new strain of ''Cyanobacteria'' carrying alkane-producing ''Membrane Accelerator'', which could produce and export alkane effectively just by utilizing carbon dioxide and light. Thus the energy crisis would no longer be a problem. | ||
| + | |||
| + | ==Reference== | ||
| + | 1.Schirmer, A., M. A. Rude, et al. (2010). "Microbial biosynthesis of alkanes." Science 329(5991): 559-562 | ||
| + | |||
| + | 2.Yu, X., T. Liu, et al. (2011). "In vitro reconstitution and steady-state analysis of the fatty acid synthase from Escherichia coli." Proceedings of the National Academy of Sciences 108(46): 18643-18648. | ||
{{Template:12SJTU_footer}} | {{Template:12SJTU_footer}} | ||
Latest revision as of 03:54, 27 October 2012
| ||
|
 "
"