|
|
| (66 intermediate revisions not shown) |
| Line 1: |
Line 1: |
| | <!-- Include the next line at the beginning of every page --> | | <!-- Include the next line at the beginning of every page --> |
| | {{:Team:LMU-Munich/Templates/Page Header|File:Team-LMU_streaked_plate.resized.jpg|3}} | | {{:Team:LMU-Munich/Templates/Page Header|File:Team-LMU_streaked_plate.resized.jpg|3}} |
| - | [[File:GerminationSTOP_banner.jpg|620px|link=]] | + | [[File:GerminationSTOP_bannerii.jpg|620px|link=]] |
| | | | |
| | | | |
| - | [[File:GerminationSTOP.png|100px|right|link=Team:LMU-Munich/Germination_Stop]] | + | [[File:GerminationSTOP.png|100px|right|link=]] |
| | + | |
| | | | |
| | | | |
| | == '''Germination'''STOP == | | == '''Germination'''STOP == |
| | + | <br> |
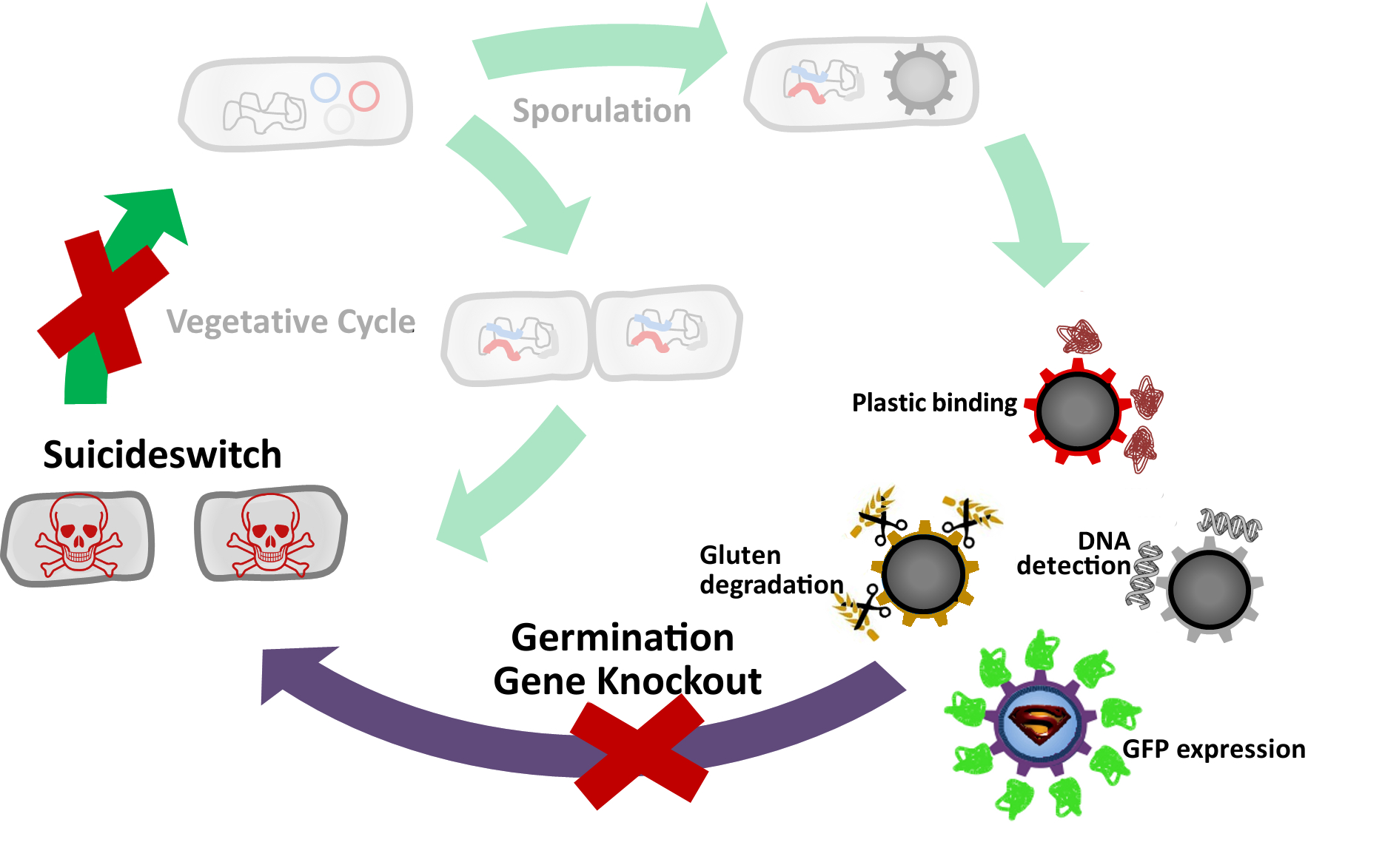
| | + | <p align="justify">The goal of this module was to yield [https://2012.igem.org/Team:LMU-Munich/Spore_Coat_Proteins '''Sporo'''beads] which are safe (unable to germinate) and consistently functional (maintain their spore shape and structure throughout time). To achieve this, we sought to remove the germination capability of our spores, while keeping their necessary structural functions intact.</p> |
| | | | |
| - | <p align="justify">The goal of this subproject was to yield [https://2012.igem.org/Team:LMU-Munich/Spore_Coat_Proteins '''Sporo'''beads] which are safe (unable to germinate) and consistently functional (maintain their spore shape and structure). To achieve this, we sought to remove the germination capability of our spores, while keeping their necessary structural functions intact.</p>
| |
| | | | |
| | + | [[File:GerminationSTOP_cycleIIii.jpg|620px|center]] |
| | | | |
| - | Two approaches were used to achieve this:
| + | [[File:NEXT.png|right|80px|link=Team:LMU-Munich/safetytour]] [[File:BACK.png|left|80px|link=Team:LMU-Munich/Bacillus_BioBricks]] |
| | | | |
| - | * [[Team:LMU-Munich/Germination_Stop#How do Germination Gene Knockouts Work?|Knock out]] genes that are involved in germination.
| |
| | | | |
| - | * [[Team:LMU-Munich/Germination_Stop#Suicide switch|'''Suicide''' switch]]: Toxin production by vegetative cells if germination knockout fails and spores manage to germinate.
| |
| | | | |
| | | | |
| - | [[File:GerminationSTOP_cycleII.jpg|620px|center]]
| |
| | | | |
| | | | |
| - | ==How does Germination Work?==
| |
| | | | |
| - | {| style="color:black;" cellpadding="3" width="35%" cellspacing="15" border="0" align="right" style="text-align:left;"
| |
| - | | style="width: 70%;background-color: #EBFCE4;" |
| |
| - | {|
| |
| - | |[[File:sporulation_diagram.jpg|200px]]
| |
| - | |-
| |
| - | | style="width: 70%;background-color: #EBFCE4;" |
| |
| - | {| style="color:black;" cellpadding="0" width="85%" cellspacing="0" border="0" align="center" style="text-align:left;"
| |
| - | |style="width: 70%;background-color: #EBFCE4;" |
| |
| - | <font color="#000000"; size="2">Fig. 1: Taken from [http://www.ncbi.nlm.nih.gov/pubmed/19554258 Kim, J. & Schumann W (2009)]. <br />
| |
| - | '''A''': Cell at stage 0; vegetative phase. <br />
| |
| - | '''B''': Cell at stage II; asymmetric septum has been formed. <br />
| |
| - | '''C''': Cell at stage III; cytoplasmic membrane has engulfed forespore. <br />
| |
| - | '''D''': Cell at stage IV; coat formation has started; spore will be released from lysed mother cell.</font>
| |
| - | |}
| |
| - | |}
| |
| - | |}
| |
| | | | |
| - | <p align="justify">The ''Bacillus'' life cycle can include both classic division, and also reproduction by sporulation and spore germination.
| |
| - | In response to starvation of nutrients (including carbon, nitrogen, or phosphorus) or in response to peptides secreted by other cells which signal too high of population densities to cells, ''Bacillus'' cells form spores in a process called sporulation.
| |
| - | The “mother” cell forms the endospore within its own cell membrane. The endospore contains its DNA in the spore core, which is protected by several layers of coats. The outermost layer is the spore crust. The spore is very dry, and contains a substance called dipicolinic acid (DPA), which is replaced with water when the spore germinates. Until the spore hydrates and swells out of its protective coats, it is resistant to a wide variety of environmental stressors, including UV radiation, toxic chemicals, freezing, high heat, dessication, and pH extremes. This resistance to stressors allows the spore to survive until conditions are good for growth.</p>
| |
| - | <p align="justify">On its inner spore membrane, the spore has germinant receptors. The spore coats are believed to be semipermeable or porous, in order to permit the passage of germinants to the receptors. When germinants such as amino acids and sugars reach germinant receptors, the spore begins a biochemical process of germination. It takes up water, shifts its pH, and swells. It breaks out of its coat and begins the outgrowth process (see Fig. 2). We wish to prevent the germination process.</p>
| |
| | | | |
| - | {| style="color:black;" cellpadding="3" width="70%" cellspacing="0" border="0" align="center" style="text-align:left;"
| |
| - | | style="width: 70%;background-color: #EBFCE4;" |
| |
| - | {|align:center
| |
| - | |[[File:germination_from_spore.jpg|620px|center]]
| |
| - | |-
| |
| - | | style="width: 80%;background-color: #EBFCE4;" |
| |
| - | {| style="color:black;" cellpadding="3" width="95%" cellspacing="0" border="0" align="left" style="text-align:left;"
| |
| - | |style="width: 70%;background-color: #EBFCE4;" |
| |
| - | <font color="#000000"; size="2">Fig. 2: '''The germination process in ''Bacillus'' spores.''' Taken from [http://www.ncbi.nlm.nih.gov/pubmed/14662349 Setlow 2003]. </font>
| |
| - | |}
| |
| - | |}
| |
| - | |}
| |
| | | | |
| | | | |
| - | ==How do Germination Gene Knockouts Work?== | + | <div class="box"> |
| - | | + | ==Gene Knockouts== |
| - | <p align="justify">Based on the work of others, we chose to knock out genes ''cwlJ'', ''sleB'', ''cwlB'', ''gerD'', and ''cwlD''. Past works showed:</p>
| + | {| "width=100%" style="text-align:center;" style="align:right"| |
| - | *'''''cwlJ'' and ''sleB''''': both genes code for lytic enzymes which are active in the process of germination. When knocked out together, germination frequency was reduced by 5 orders of magnitude.
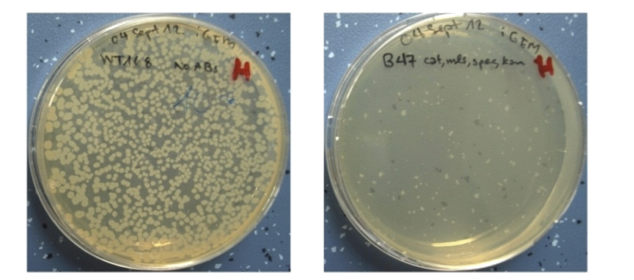
| + | |<p align="justify">We picked important germination genes and knocked them out. Subsequently we combined the single mutants into quadruple mutants and successfully prevented germination.</p> |
| - | *'''''gerD'' and ''cwlB''''': the ''gerD'' product plays unknown role in nutrient germination; ''cwlB'''s product plays role in cell wall turnover & cell lysis. When knocked out together, germination frequency was reduced by 5 orders of magnitude.
| + | |[[File:LMU Germination STop plate.png|200px|right|link=Team:LMU-Munich/Germination_Stop/Knockout]] |
| - | *'''''cwlD''''': this gene codes for recognition components for cleavage by the germination-specific cortex lytic enzymes. When knocked out, germination occurred at a rate of 0.003 to 0.05%.
| + | |
| - | <p align="justify">By knocking out all five of these genes, our goal was to yield a ''B. subtilis'' strain which produces spores completely incapable of germination. The stop to germination comes during the process of spore coat breakdown. Without the lytic enzymes to break down the coat, the spores should be unable to outgrow into the vegetative stage. | + | |
| - | After several attempts to knock out ''cwlB'', and producing extremely slow-growing mutants, ''cwlB'' was removed from our list of genes to knock out.</p>
| + | |
| - | | + | |
| - | {| class="colored"
| + | |
| - | !Germination Genes
| + | |
| - | !Gene Function
| + | |
| - | !Mutant Germination Rate
| + | |
| - | |- | + | |
| - | !''gerD''
| + | |
| - | |Unknown role in nutrition germination | + | |
| - | |Reduction by 5 orders of magnitude
| + | |
| - | |- | + | |
| - | !''cwlJ''
| + | |
| - | |Germination-specific lytic enzymes
| + | |
| - | |0.003 – 0.05%
| + | |
| - | No ATP detected
| + | |
| - | |-
| + | |
| - | !''sleB''
| + | |
| - | |Germination-specific lytic enzymes
| + | |
| - | |0.003 – 0.05%
| + | |
| - | No ATP detected
| + | |
| | |- | | |- |
| - | !''cwlD'' | + | ! colspan="2" |[[File:LMU Arrow purple.png|40px|link=Team:LMU-Munich/Germination_Stop/Knockout]] |
| - | |Recognition component for lytic enzymes | + | |
| - | |0.003 – 0-005%
| + | |
| | |} | | |} |
| | + | </div> |
| | | | |
| - | | + | <div class="box"> |
| - | | + | =='''Suicide'''switch== |
| - | ==What Methods Did We Use to Knockout Germination Genes?==
| + | {| "width=100%" style="text-align:center;" style="align:right"| |
| - | | + | |<p align="justify">In case of germination gene knockout failure, we invented the suicide switch. If spores still germinate, the production of a toxin leads to immediate cell death.</p> |
| - | <p align="justify">Two methods were employed to knock out germination: resistance cassette knockouts and clean deletions. Resistance cassette (RC) knockouts were performed using long-flanking-homology PCR (see Fig. 3 and [https://2012.igem.org/Team:LMU-Munich/Lab_Notebook/Protocols Protocols]). Single RC knockouts were created first; then they were combined to create multiple knockouts. The genome with all RC knockouts can be seen in Fig 4.</p> | + | |[[File:LMU SuicideSwitch grafik.png|200px|right|link=Team:LMU-Munich/Germination_Stop/SuicideSwitch]] |
| - | | + | |
| - | {| style="color:black;" cellpadding="3" width="70%" cellspacing="0" border="0" align="center" style="text-align:left;" | + | |
| - | | style="width: 70%;background-color: #EBFCE4;" |
| + | |
| - | {|align:center
| + | |
| - | |[[File:lfhPCR.jpg|620px|center]] | + | |
| | |- | | |- |
| - | | style="width: 80%;background-color: #EBFCE4;" |
| + | ! colspan="2" |[[File:LMU Arrow purple.png|40px|link=Team:LMU-Munich/Germination_Stop/SuicideSwitch]] |
| - | {| style="color:black;" cellpadding="3" width="95%" cellspacing="0" border="0" align="left" style="text-align:left;"
| + | |
| - | |style="width: 70%;background-color: #EBFCE4;" | | + | |
| - | <font color="#000000"; size="2">Fig. 3: '''Procedure of long-flanking homology PCR.''' As an example, replacement of ''cwlD'' by a kanamycin (kan) resistance cassette is shown. 1000 base-pair fragments flanking ''cwlD'' were amplified. Up-reverse and down-forward primers have overhangs complementary to kan cassette. Kan separately amplified. Up and down ''clwD'' fragments and amplified kan cassettes were put into the PCR reaction. The result is a fragment containing the kann cassette flanked by ''cwlD''. ''Bacillus subtilis'' is transformed with the fragment; replacement is checked by PCR.</font>
| + | |
| | |} | | |} |
| - | |}
| + | </div> |
| - | |}
| + | <br> |
| - | | + | <br> |
| - | | + | <br> |
| - | {| style="color:black;" cellpadding="3" width="70%" cellspacing="0" border="0" align="center" style="text-align:left;"
| + | <br> |
| - | | style="width: 70%;background-color: #EBFCE4;" |
| + | |
| - | {|align:center
| + | |
| - | |[[File:germination_gene_knockouts_circle.jpg|620px|center]]
| + | |
| - | |-
| + | |
| - | | style="width: 80%;background-color: #EBFCE4;" |
| + | |
| - | {| style="color:black;" cellpadding="3" width="95%" cellspacing="0" border="0" align="left" style="text-align:left;"
| + | |
| - | |style="width: 70%;background-color: #EBFCE4;" |
| + | |
| - | <font color="#000000"; size="2">Fig. 4: The four germination genes being knocked out, and their resistance cassette replacements, as shown on the ''Bacillus'' chromosome.</font>
| + | |
| - | |}
| + | |
| - | |}
| + | |
| - | |}
| + | |
| - | | + | |
| - | | + | |
| - | <p align="justify">The germination rate of our mutants were checked with a [[Team:LMU-Munich/Lab_Notebook/Protocols|germination assay]]. The assay was developed based on the protocols of other researchers.
| + | |
| - | | + | |
| - | In the event that some small percentage of spores retained the ability to germinate, the '''Suicide''' switch subproject (below) prevents outgrowth into viable vegetative cells.</p>
| + | |
| - | | + | |
| - | | + | |
| - | =='''Suicide''' switch==
| + | |
| - | | + | |
| - | | + | |
| - | {| style="color:black;" cellpadding="3" width="70%" cellspacing="0" border="0" align="center" style="text-align:left;"
| + | |
| - | | style="width: 70%;background-color: #EBFCE4;" |
| + | |
| - | {|
| + | |
| - | |[[File:LMU SuicideSwitch grafik.png|624px|center]]
| + | |
| - | |-
| + | |
| - | | style="width: 70%;background-color: #EBFCE4;" |
| + | |
| - | {| style="color:black;" cellpadding="0" width="70%" cellspacing="0" border="0" align="center" style="text-align:center;"
| + | |
| - | |style="width: 70%;background-color: #EBFCE4;" |
| + | |
| - | <font color="#000000"; size="2">Fig. 5: Genetic elements of the '''Suicide''' switch and its expected performance.</font>
| + | |
| - | |}
| + | |
| - | |}
| + | |
| - | |}
| + | |
| - | | + | |
| - | | + | |
| - | <p align="justify">As a backup plan to make our <b>Sporo</b>beads even safer, we developed the <b>Suicide</b>switch. In case the spores do germinate, due to degradation or destruction of their outer coats, e.g. by high pressure, the <b>Suicide</b>switch will be turned on.<br></p>
| + | |
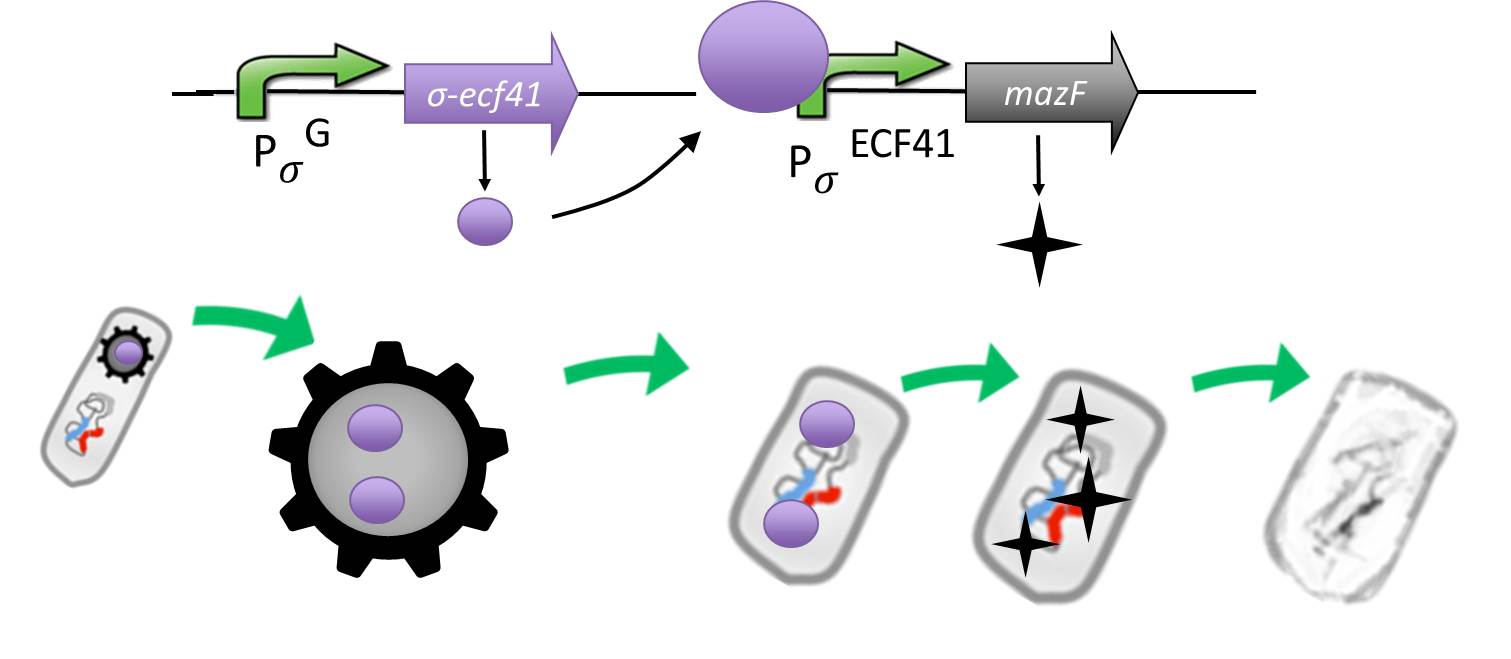
| - | <p align="justify">It is composed by an alternative sigma factor [http://partsregistry.org/wiki/index.php?title=Part:BBa_K823043 ECF41] ([http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3426412/ Wecke T., Mascher T. 2012]), which derives from ''B. lincheniformes'' and we chose to work with the short version constitutively on. It is synthetically linked to a sigma G regulated promotor responding quite late to sigma G ([http://partsregistry.org/wiki/index.php?title=Part:BBa_K823048 PspoIVB] ([http://www.ncbi.nlm.nih.gov/pubmed/15699190 Steil L., Völker U. et al. 2005]) responding strongly or [http://partsregistry.org/wiki/index.php?title=Part:BBa_K823042 PsspK] ([http://www.ncbi.nlm.nih.gov/pubmed/15699190 Steil L., Völker U. et al. 2005]) responding weakly) which is the last sigma factor activated in the forespore. Through this ecf41 is produced quite late in the forespore. Ecf41 then activates the [http://partsregistry.org/wiki/index.php?title=Part:BBa_K823041 PydfG] ([http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3426412/ Wecke T., Mascher T. 2012]) promotor, which is the naturally responding promoter to ECF41, which then activates the transcription of [http://partsregistry.org/wiki/index.php?title=Part:BBa_K823044 MazF] ([http://jcs.biologists.org/content/118/19/4327.abstract Engelberg-Kulka H.,Amitai S. 2005]), a bacterial toxin from ''E.coli'' degrading mRNA. <br></p>
| + | |
| - | <p align="justify">The idea behind this is to pack the <b>Sporo</b>beads full with ECF41 when they sporulate, which will in turn kill them upon germination due to the MazF. <br></p>
| + | |
| - | <p align="justify">Through the system with the alternative sigma factor the forespore gains hopefully enough time to fully mature before MazF starts to be produced.<br></p>
| + | |
| - | <p align="justify">We chose MazF, as we think it will not be able to harm our <b>Sporo</b>bead even if it is made too early, as the spore does not rely on translation to be preserved. <br></p>
| + | |
| - | See what [https://2012.igem.org/Team:LMU-Munich/Data/Suicideswitch Data] we get when measuring a module with ''luxABCDE'' instead of MazF.
| + | |
| - | | + | |
| - | We are planning to model this system, but still need some Data for it, including such which are first possible to get after cloning PsspK and MazF.
| + | |
| - | | + | |
| - | | + | |
| - | | + | |
| - | | + | |
| - | | + | |
| - | | + | |
| - | | + | |
| | <div class="box"> | | <div class="box"> |
| | ====Project Navigation==== | | ====Project Navigation==== |







 "
"