Team:Kyoto/Secretion/Notebook
From 2012.igem.org
(→September 20) |
(→Secretion Notebook) |
||
| (52 intermediate revisions not shown) | |||
| Line 4: | Line 4: | ||
<div class="_kyoto-note"> | <div class="_kyoto-note"> | ||
==February 7== | ==February 7== | ||
| - | + | ====Preculture==== | |
| - | We started preculture at 12:10.<br> | + | We started preculture at 12:10.<br> |
| + | |||
==February 8== | ==February 8== | ||
| - | + | ====Culture==== | |
We start culturing with 300[mL] of LB medium.<br> | We start culturing with 300[mL] of LB medium.<br> | ||
{|class="wikitable" | {|class="wikitable" | ||
| Line 22: | Line 23: | ||
|15:21||0.64 | |15:21||0.64 | ||
|} | |} | ||
| - | + | ====Making Competent Cell==== | |
We made competent cells.<br><br> | We made competent cells.<br><br> | ||
| - | + | ====Transformation==== | |
| - | pGEM_TAP<br> | + | pGEM_TAP<br>LacP (BBa_R0011)<br>DT (BBa_B0015)<br><br> |
| - | + | ====Making Culture Medium Plates==== | |
We made 200mL of ampicillin culture, kanamycin culture, and chloramphenicol culture.<br><br> | We made 200mL of ampicillin culture, kanamycin culture, and chloramphenicol culture.<br><br> | ||
| - | + | ====Transformation==== | |
| - | GFP(BBa_E0040) in pSB1A2<br>DT(BBa_B0015) in pSB1AK3<br>ara(BBa_I0500) in pSB2K3<br> | + | GFP(BBa_E0040) in pSB1A2<br>DT(BBa_B0015) in pSB1AK3<br>ara(BBa_I0500) in pSB2K3<br>LacP(BBa_R0011) in pSB1A2 |
| + | |||
==February 9== | ==February 9== | ||
| - | + | ====transformation==== | |
BBa-E0040(GFP)(Mr.Fujita)<br><br> | BBa-E0040(GFP)(Mr.Fujita)<br><br> | ||
| - | + | ====Liquid culture==== | |
DT.leap colony transformed on February 8<br> | DT.leap colony transformed on February 8<br> | ||
| - | colony of competent cell made on February 8<br> | + | colony of competent cell made on February 8<br> |
| + | |||
==February 10== | ==February 10== | ||
| - | + | ====Miniprep==== | |
DT2 43.9μg/ml(1.34 260/230 1.74 260/280)<br> | DT2 43.9μg/ml(1.34 260/230 1.74 260/280)<br> | ||
| - | + | LacP1 17.1μg/ml(1.54 260/280 0.83 260/230)<br> | |
| - | + | LacP2 18.0μg/ml(1.58 260/280 0.87 260/230)<br><br> | |
| - | + | ====transformation==== | |
B0040 1.4k PsB1A2 B0034 1.2M pSB1A2(from iGEM parts plate)<br><br> | B0040 1.4k PsB1A2 B0034 1.2M pSB1A2(from iGEM parts plate)<br><br> | ||
| - | + | ====Competent cell==== | |
We did preculture for overnight. We put 1.5mL of preculture on 150mL of LB culture. | We did preculture for overnight. We put 1.5mL of preculture on 150mL of LB culture. | ||
{|class="wikitable" | {|class="wikitable" | ||
| Line 61: | Line 64: | ||
|- | |- | ||
|at last||0.576 | |at last||0.576 | ||
| - | |} | + | |} |
| + | |||
==February 11== | ==February 11== | ||
| - | + | ====Checking Transformation efficiency==== | |
| - | Conpetent cell's transformation efficiency is 1.3x10^4colonys/μg<br><br> | + | Conpetent cell's transformation efficiency is 1.3x10^4colonys/μg<br><br> |
| + | |||
==February 13== | ==February 13== | ||
| - | + | ====Transformation==== | |
| - | Const promoter J23110,J23109,J23100<br> | + | Const promoter J23110, J23109, J23100<br> |
{|class="wikitable" | {|class="wikitable" | ||
!DNA||Competent cell||total | !DNA||Competent cell||total | ||
| Line 74: | Line 79: | ||
|} | |} | ||
No colony was there on February 14<br><br> | No colony was there on February 14<br><br> | ||
| - | + | ====Liquid culture==== | |
| - | + | LacP, DT, RBS(BBa_B0034),GFP<br> | |
start at 20:00<br> | start at 20:00<br> | ||
| - | in Plus grow with Ampicilin 3mL<br> | + | in Plus grow with Ampicilin 3mL<br> |
| + | |||
==February 14== | ==February 14== | ||
| - | + | ====Miniprep==== | |
concentration[μg/μL] | concentration[μg/μL] | ||
{|class="wikitable" | {|class="wikitable" | ||
| - | | | + | |LacP3||39.6 |
|- | |- | ||
| - | | | + | |LacP4||40.8 |
|- | |- | ||
| - | | | + | |LacP5||28.9 |
|- | |- | ||
|RBS1||28.2 | |RBS1||28.2 | ||
| Line 106: | Line 112: | ||
|GFP3||66.0 | |GFP3||66.0 | ||
|} | |} | ||
| - | + | ====Restriction==== | |
Const promoterJ23100<br> | Const promoterJ23100<br> | ||
{|class="wikitable" | {|class="wikitable" | ||
| Line 113: | Line 119: | ||
|20||0.5||0.5||3||0.5||5.5||30 | |20||0.5||0.5||3||0.5||5.5||30 | ||
|} | |} | ||
| - | at 37℃ for overnight<br> | + | at 37℃ for overnight<br> |
| + | |||
==February 15== | ==February 15== | ||
| - | + | ====Making gel==== | |
1% Agarose gel<br> | 1% Agarose gel<br> | ||
{|class="wikitable" | {|class="wikitable" | ||
| Line 122: | Line 129: | ||
|1.6g||160mL | |1.6g||160mL | ||
|} | |} | ||
| - | + | ====Electrophoresis==== | |
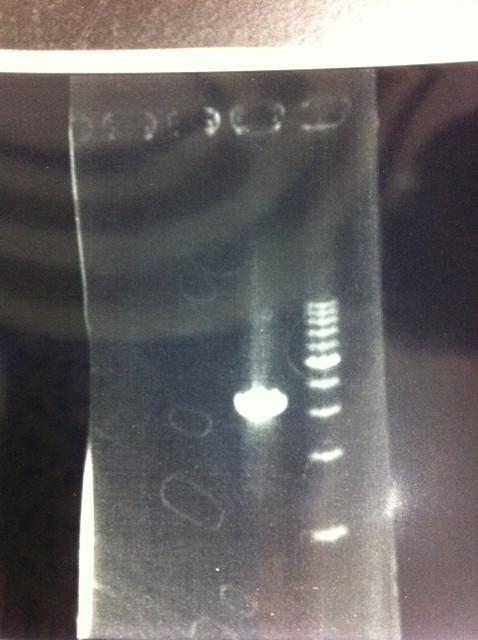
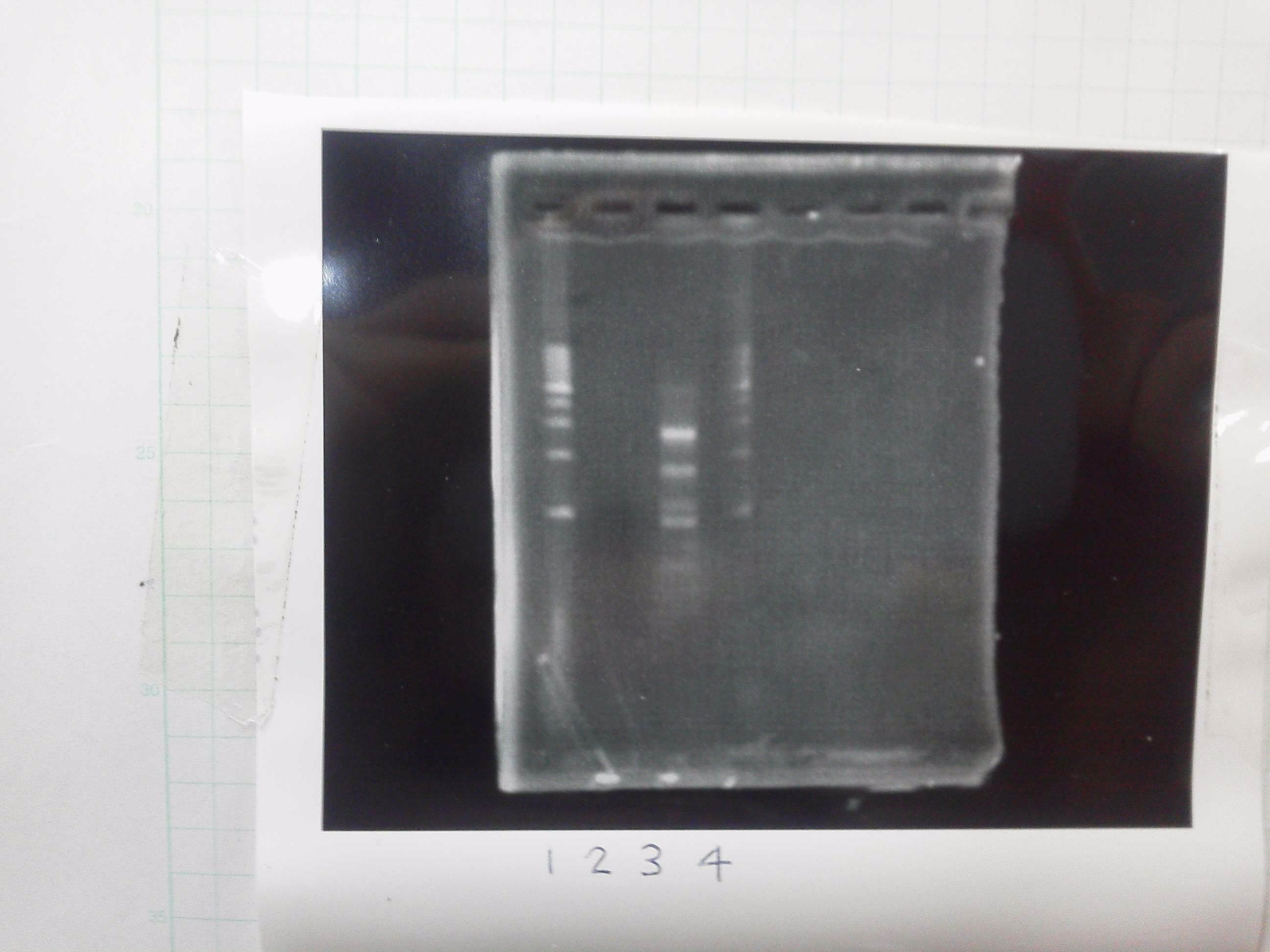
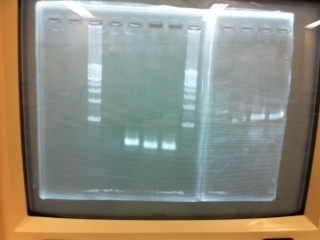
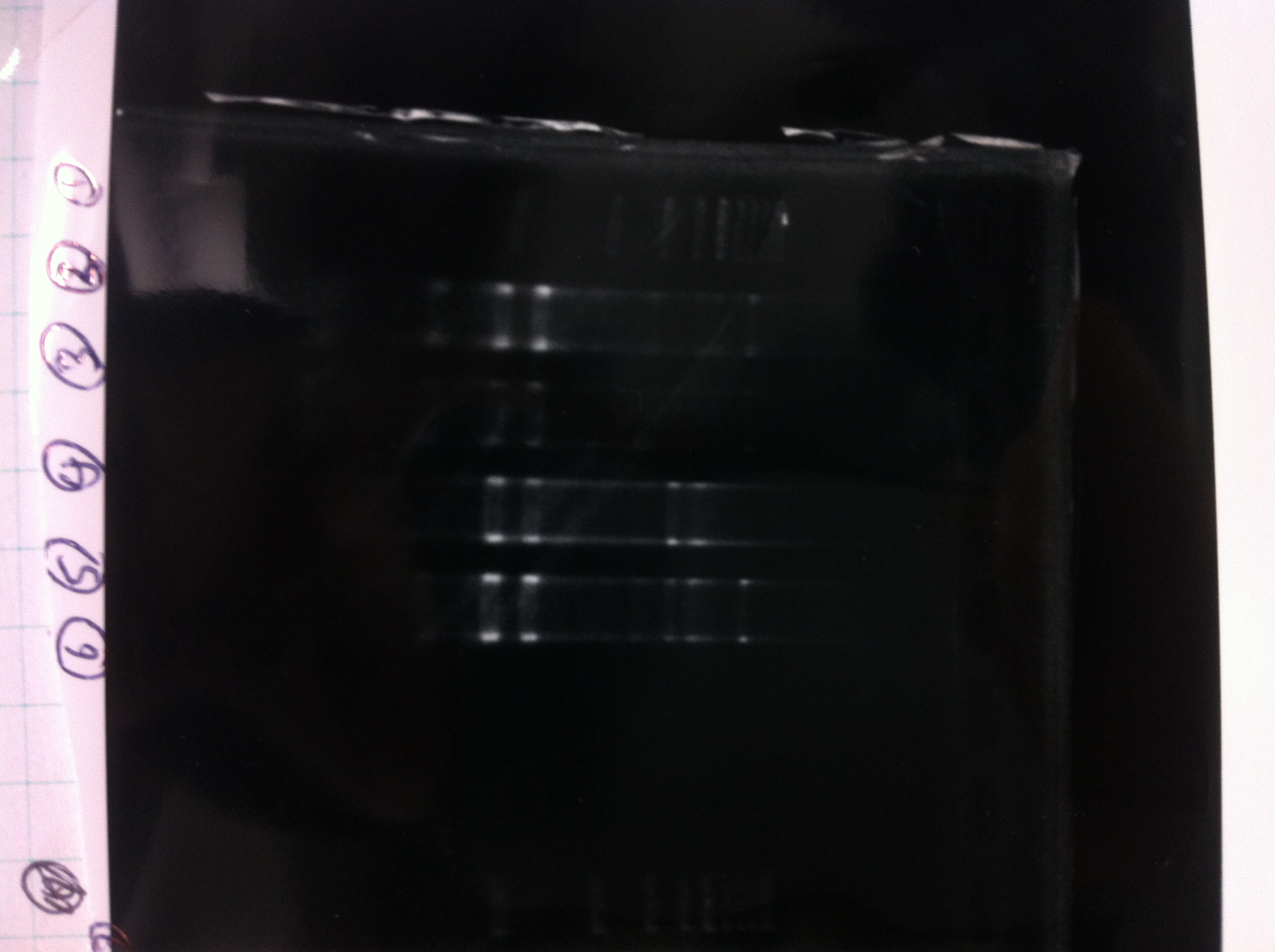
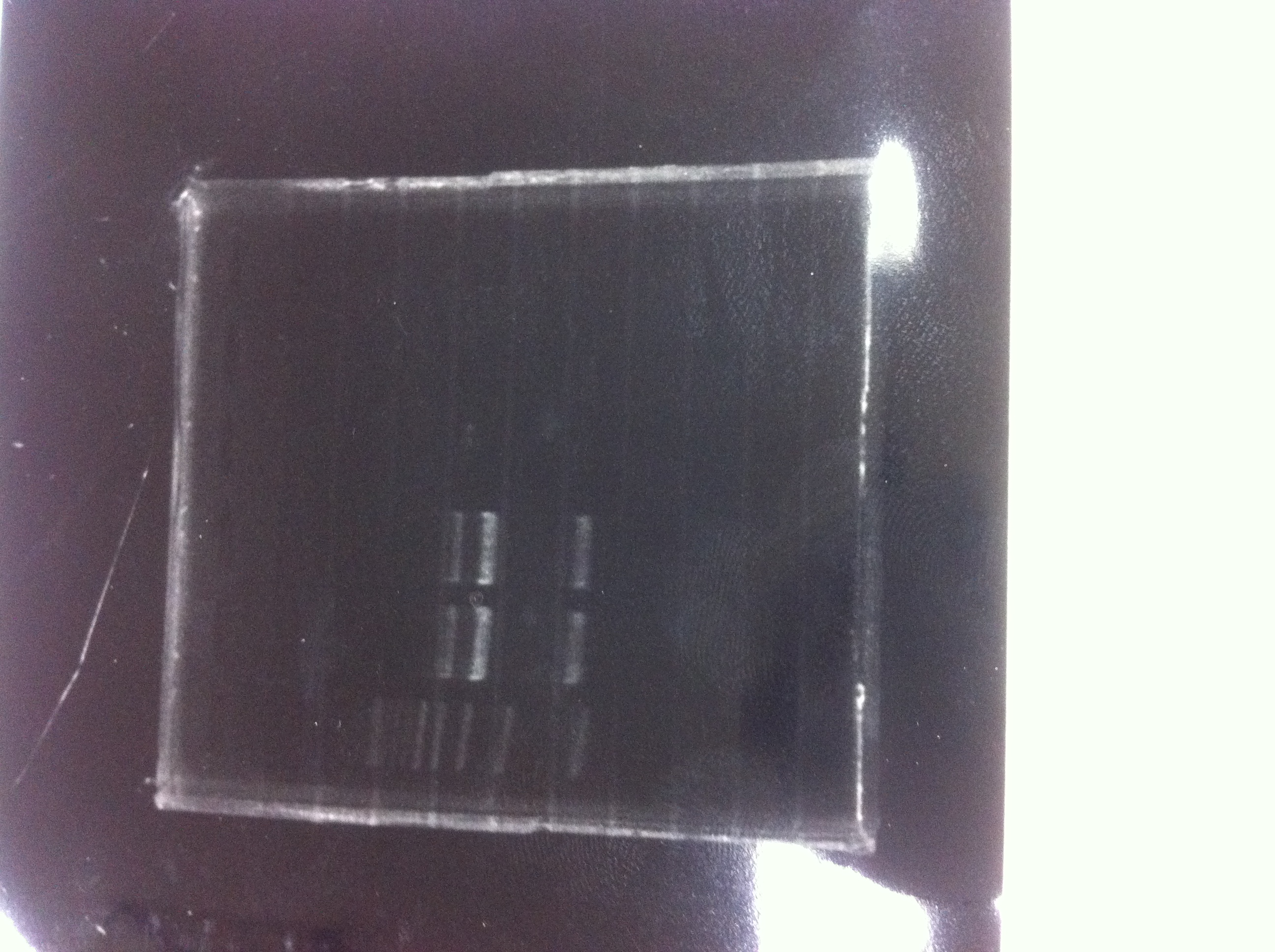
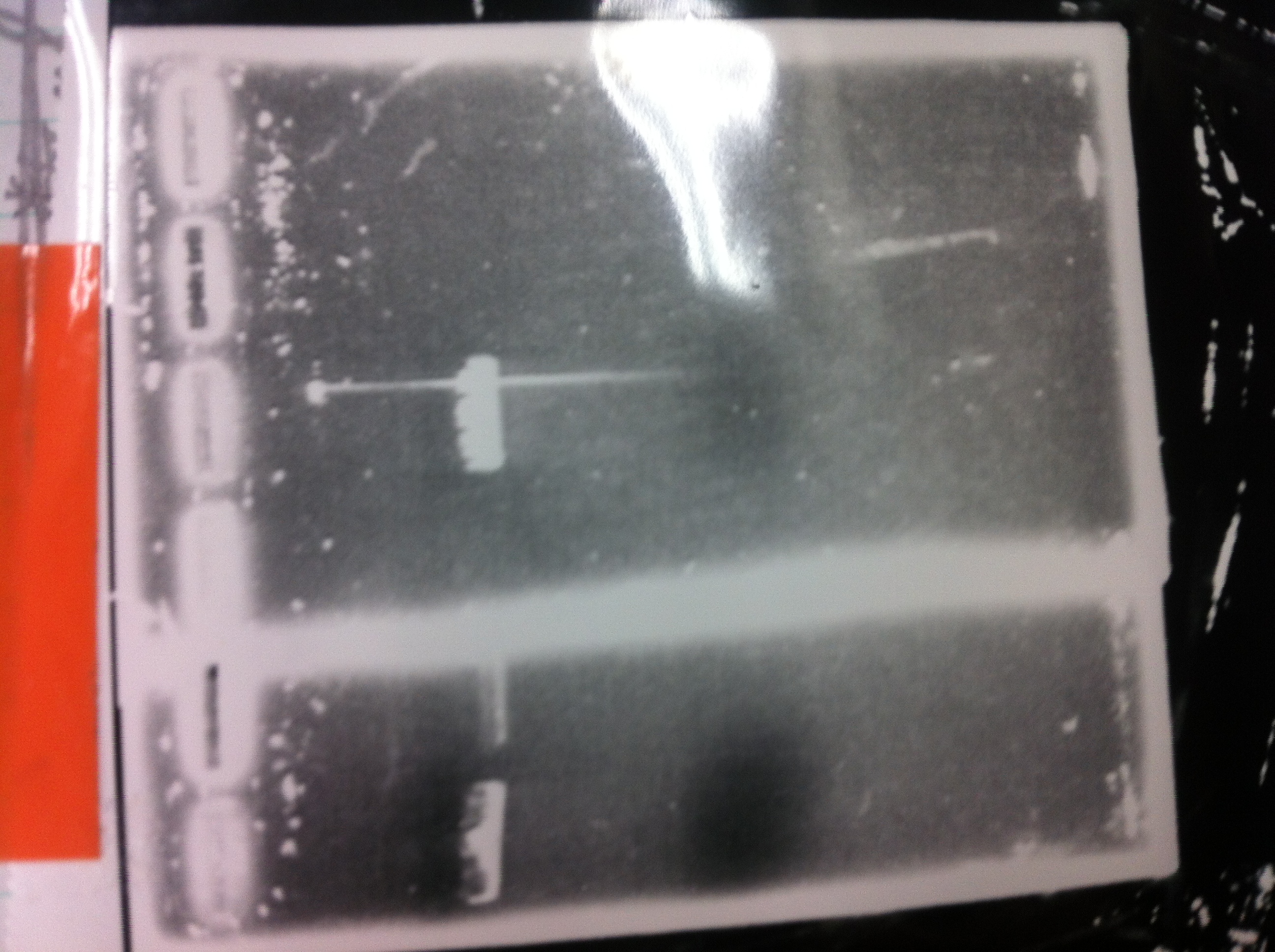
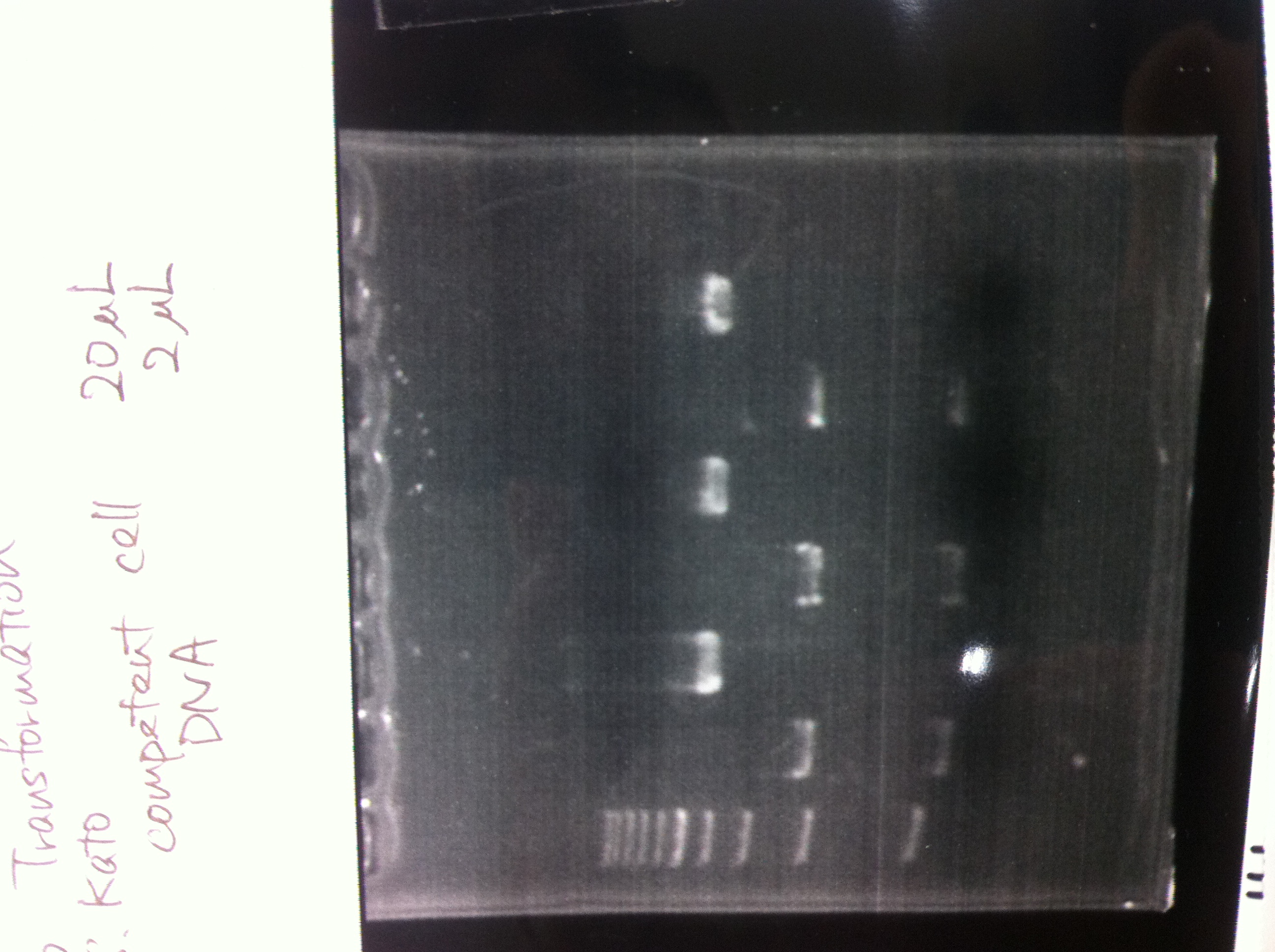
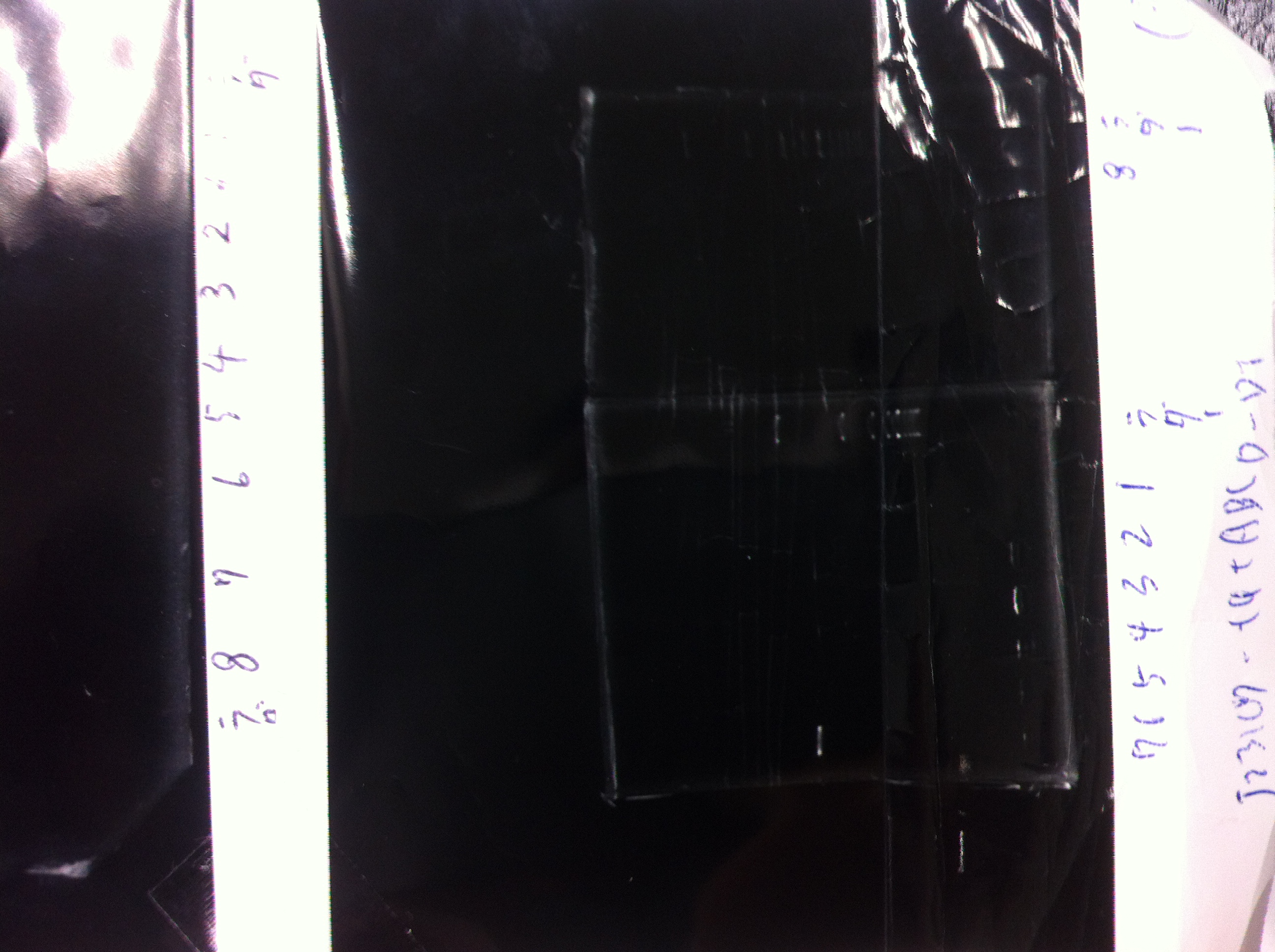
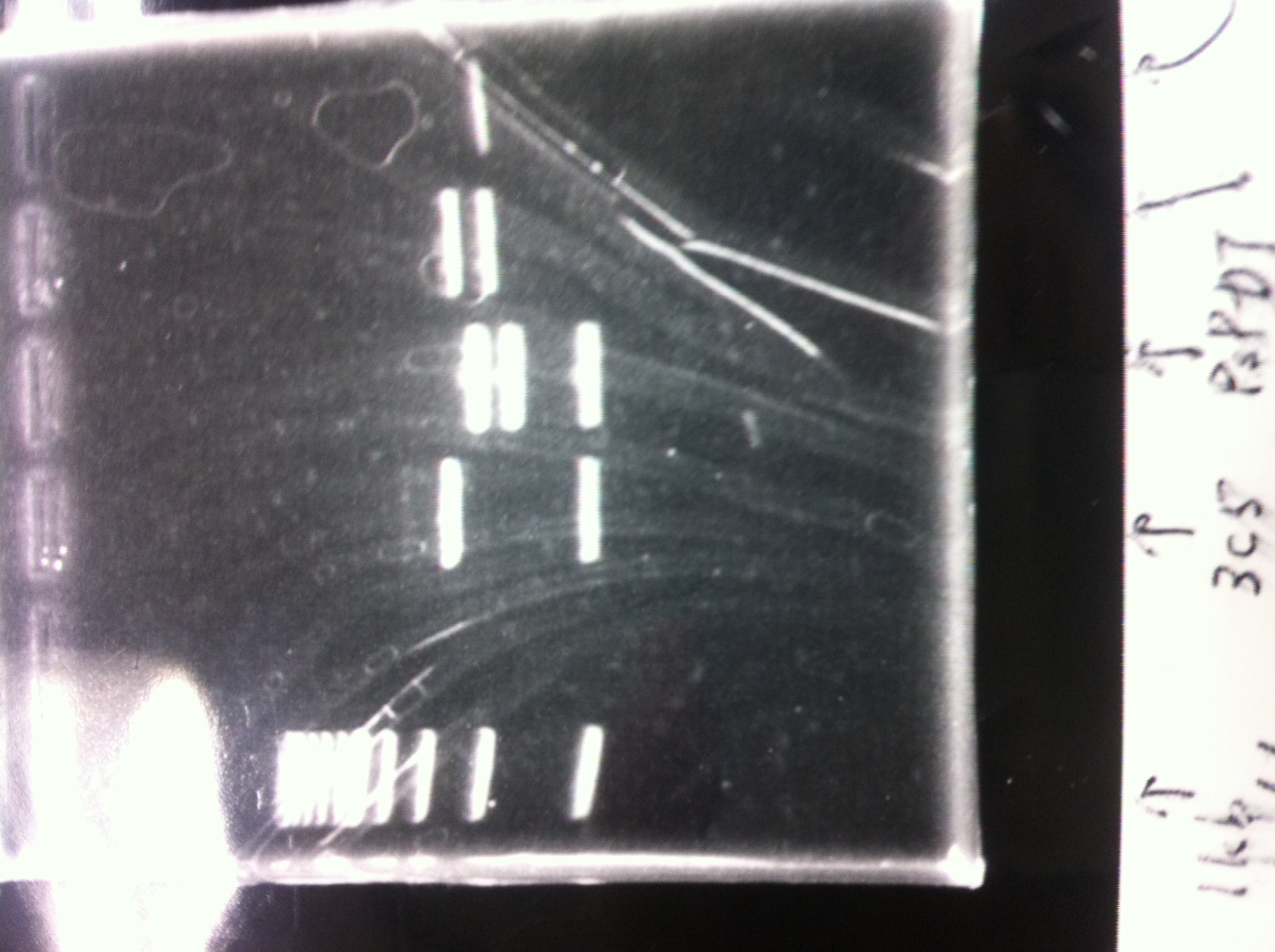
[[File:Electrophoresis021501.JPG|200px|thumb|right|No.1]] | [[File:Electrophoresis021501.JPG|200px|thumb|right|No.1]] | ||
Gel No.1 | Gel No.1 | ||
| Line 140: | Line 147: | ||
*There were bands on lane_2 and we cannot identify these bands because the sample of lane_2 was not cut with any restriction enzyme.<br> | *There were bands on lane_2 and we cannot identify these bands because the sample of lane_2 was not cut with any restriction enzyme.<br> | ||
*There must have been bands at 2100bp and 883bp on lane_3 and lane_4.<br><br> | *There must have been bands at 2100bp and 883bp on lane_3 and lane_4.<br><br> | ||
| - | + | ====Testing whether restriction enzyme were deactivated or not==== | |
{|class="wikitable" | {|class="wikitable" | ||
!DNA(DT)||restriction enzyme||Buffer||BSA||MilliQ||total | !DNA(DT)||restriction enzyme||Buffer||BSA||MilliQ||total | ||
| Line 147: | Line 154: | ||
|} | |} | ||
at 37℃ for Oveernight<br> | at 37℃ for Oveernight<br> | ||
| - | Restriction enzyme means Spe1(1,2) Pst1(1,2,3) in this time.<br><br><br><br><br><br><br><br><br><br><br><br> | + | Restriction enzyme means Spe1(1,2) Pst1(1,2,3) in this time.<br><br><br><br><br><br><br><br><br><br><br><br> |
| + | |||
==February 16== | ==February 16== | ||
| - | + | ====Electrophoresis==== | |
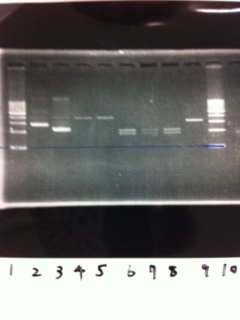
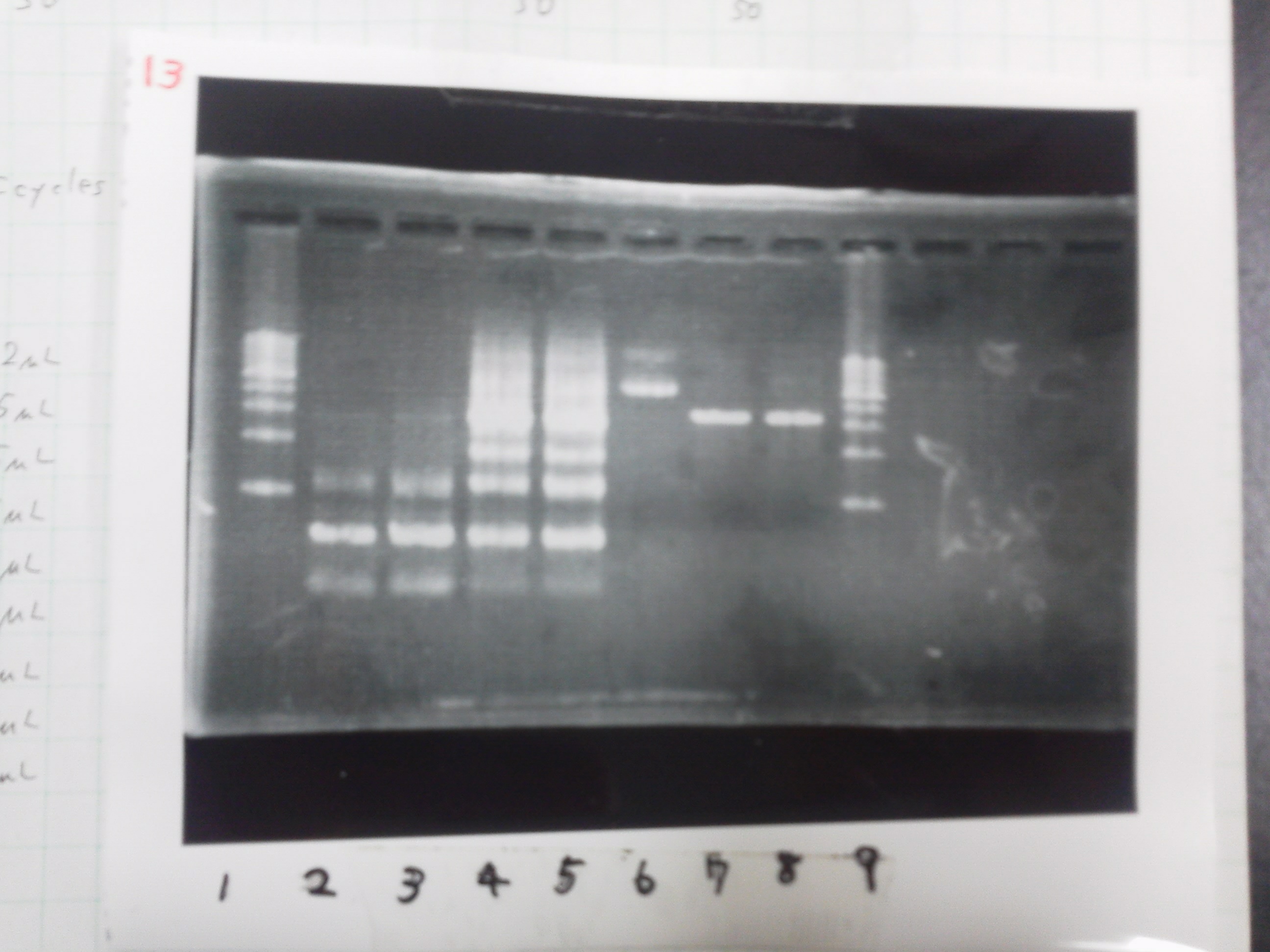
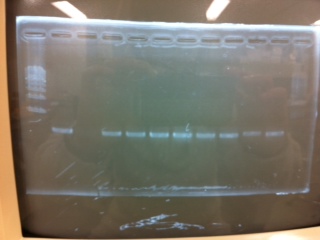
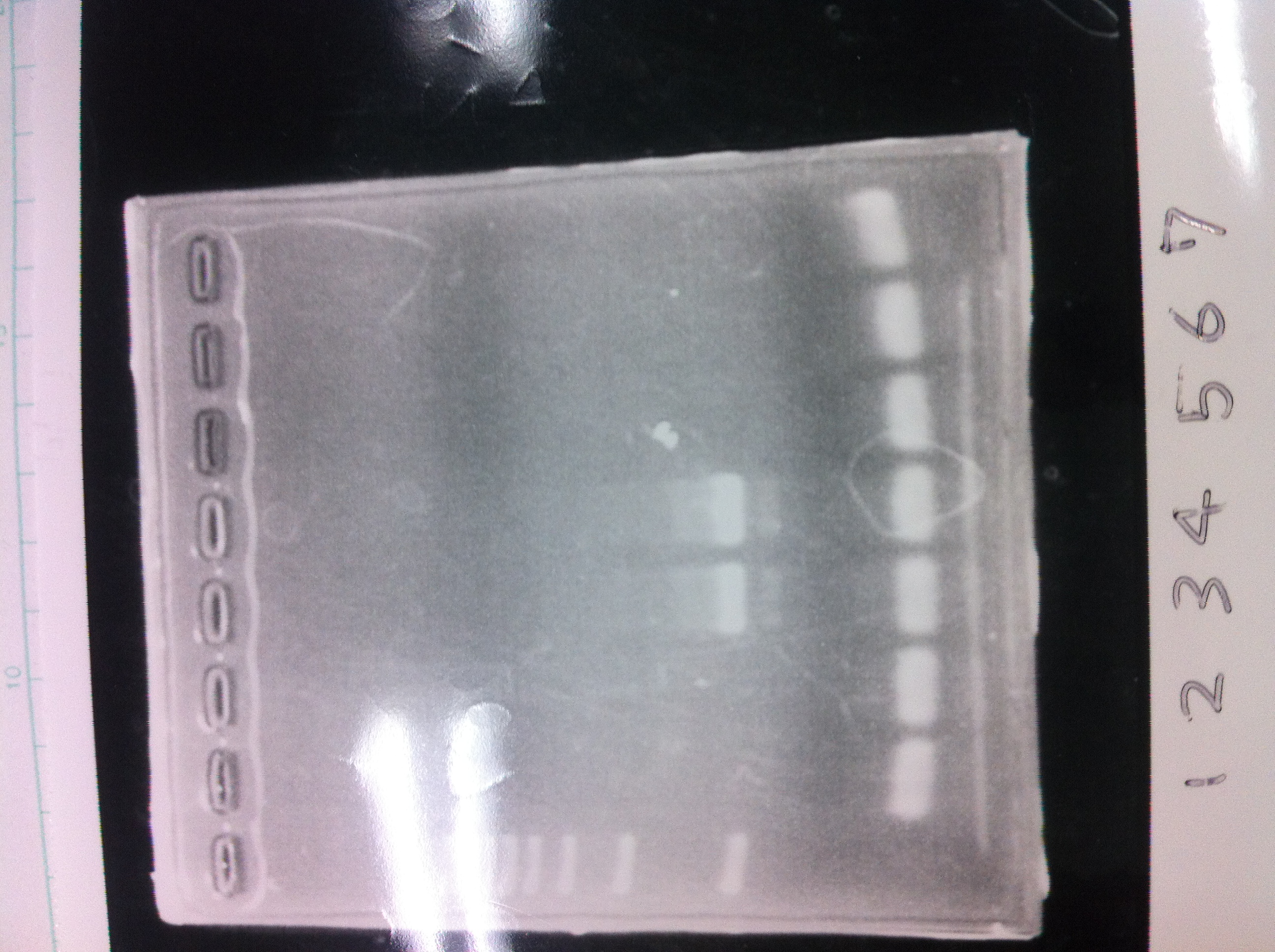
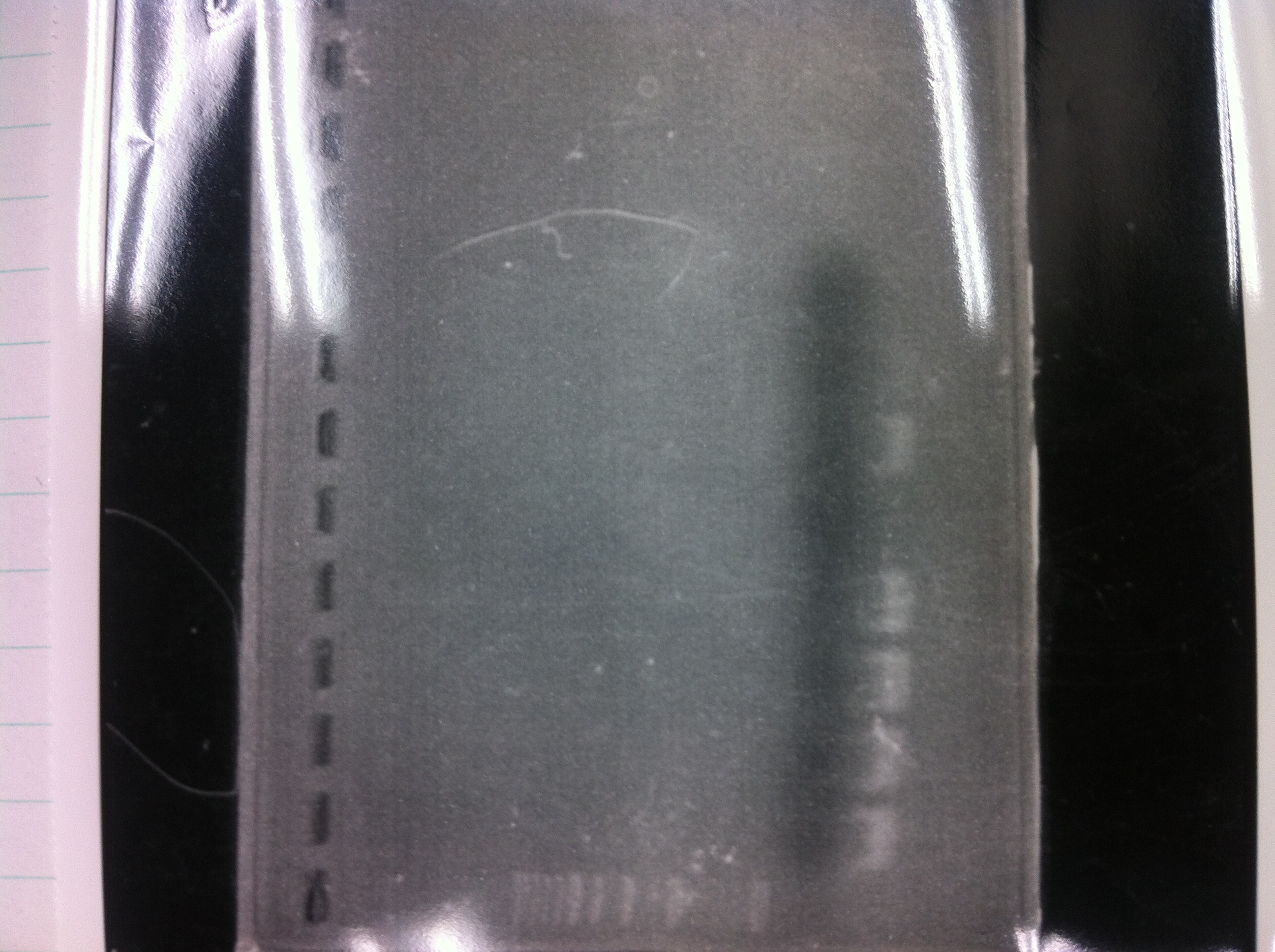
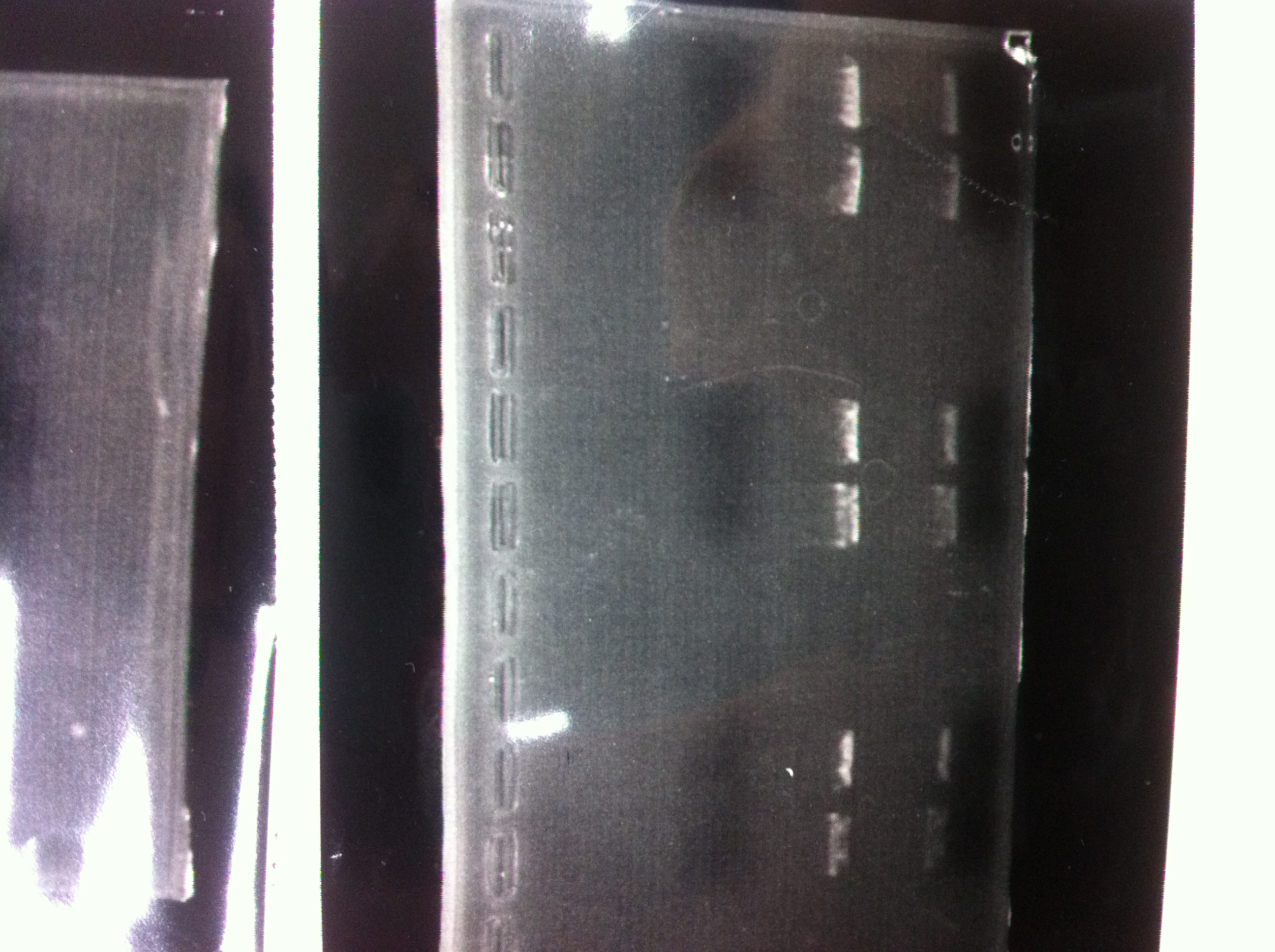
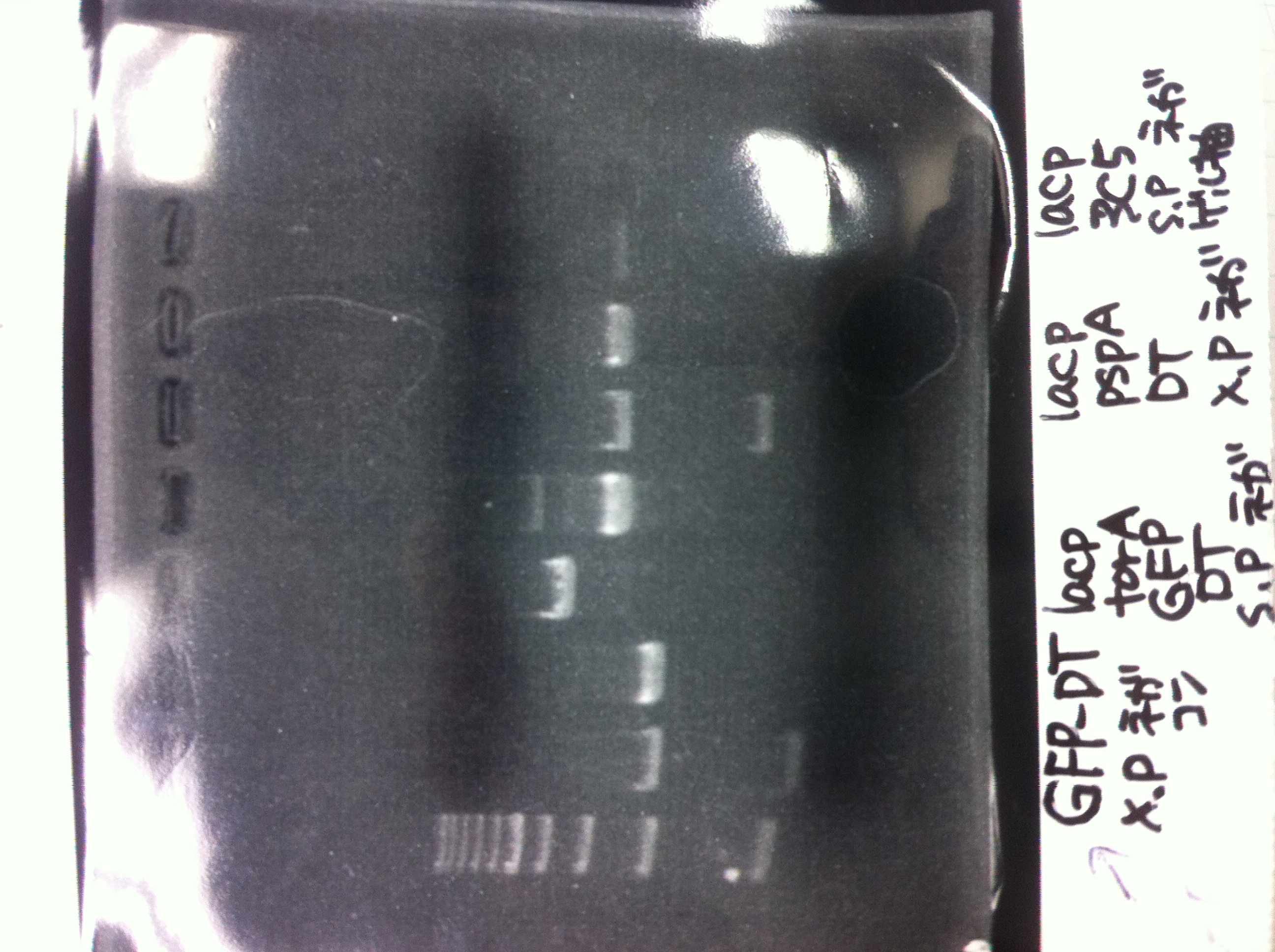
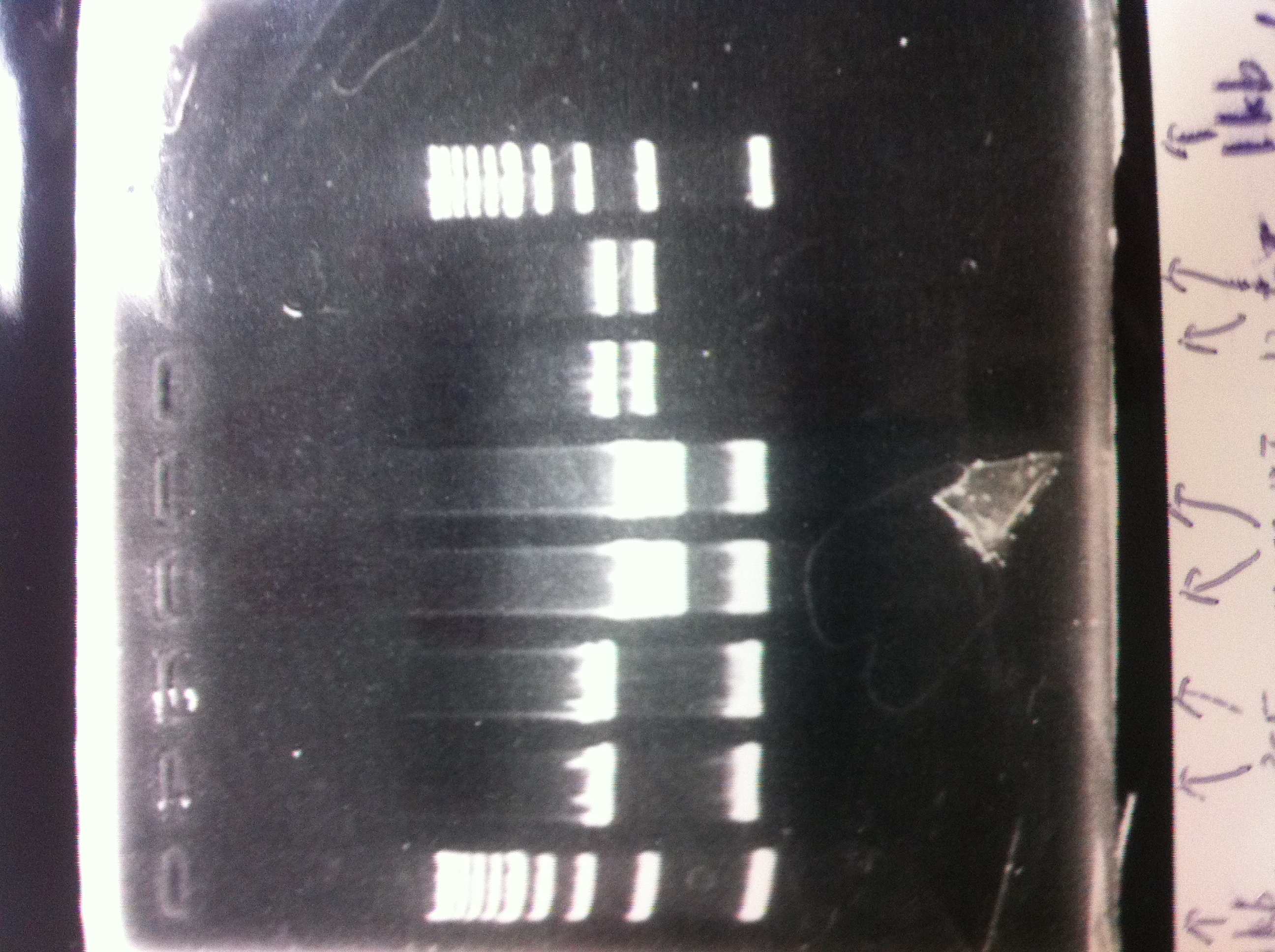
[[File:Electrophoresis021601.JPG|300px|thumb|right]] | [[File:Electrophoresis021601.JPG|300px|thumb|right]] | ||
1. 1kb ladder<br> | 1. 1kb ladder<br> | ||
| Line 162: | Line 170: | ||
10. 1kb ladder<br><br> | 10. 1kb ladder<br><br> | ||
Pst1-1, Pst1-2, and Pst1-3 did not cut DNAs. They seemed to be deactivated.<br><br> | Pst1-1, Pst1-2, and Pst1-3 did not cut DNAs. They seemed to be deactivated.<br><br> | ||
| - | + | ====Genomic PCR==== | |
{|class="wikitable" | {|class="wikitable" | ||
!10*Buffer for KOD Plus||2mM dNTPs||25mM MgSO4||10μM primer-f||10μM primer-r||158ng/μL Genomic DNA||KOD plus||MilliQ||total | !10*Buffer for KOD Plus||2mM dNTPs||25mM MgSO4||10μM primer-f||10μM primer-r||158ng/μL Genomic DNA||KOD plus||MilliQ||total | ||
| Line 168: | Line 176: | ||
|5||5||3||1.5||1.5||1||1||32||50 | |5||5||3||1.5||1.5||1||1||32||50 | ||
|} | |} | ||
| - | + | ====Electrophoresis==== | |
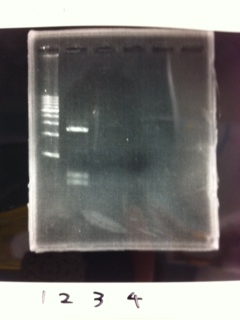
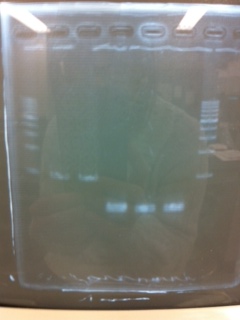
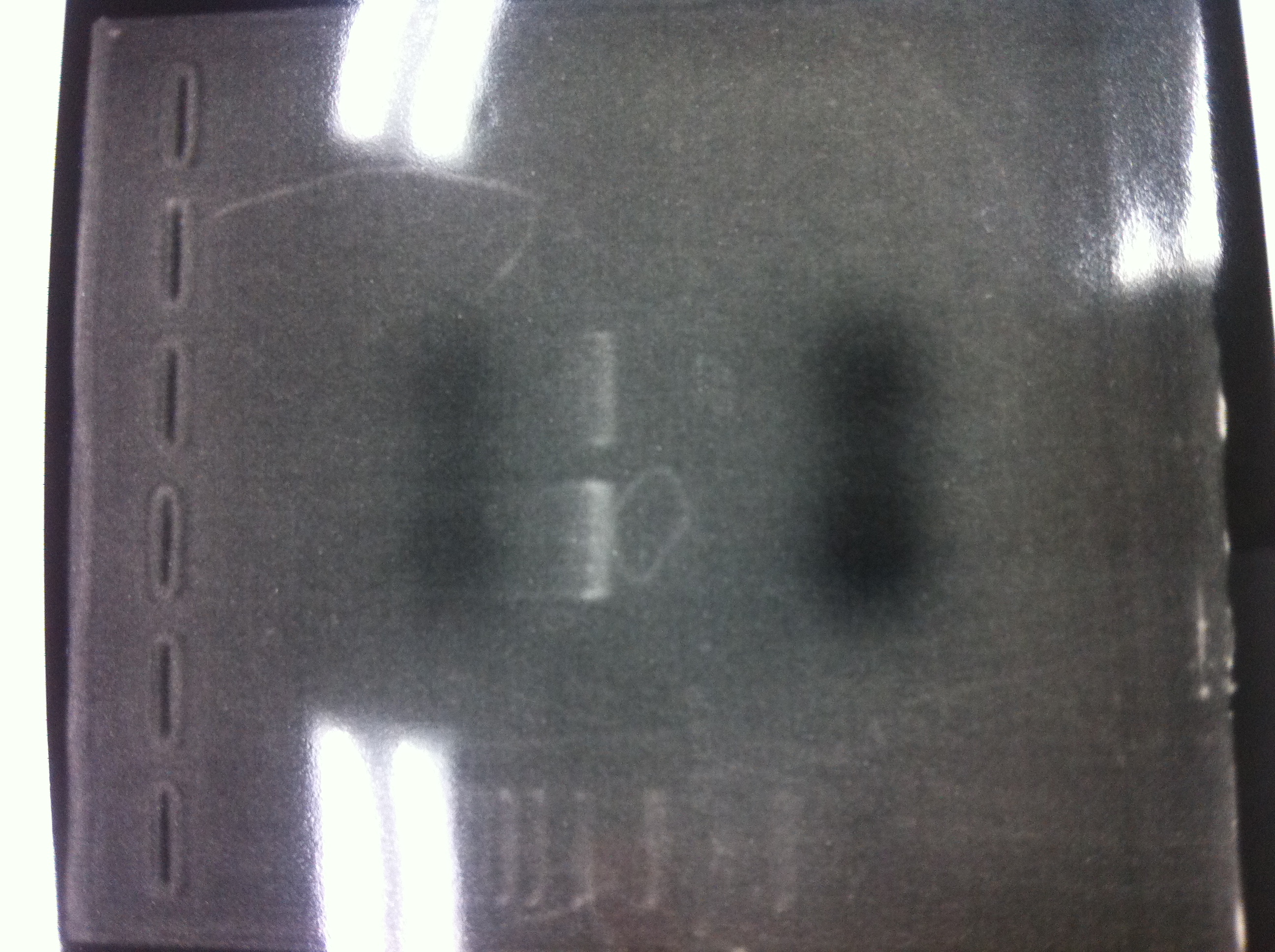
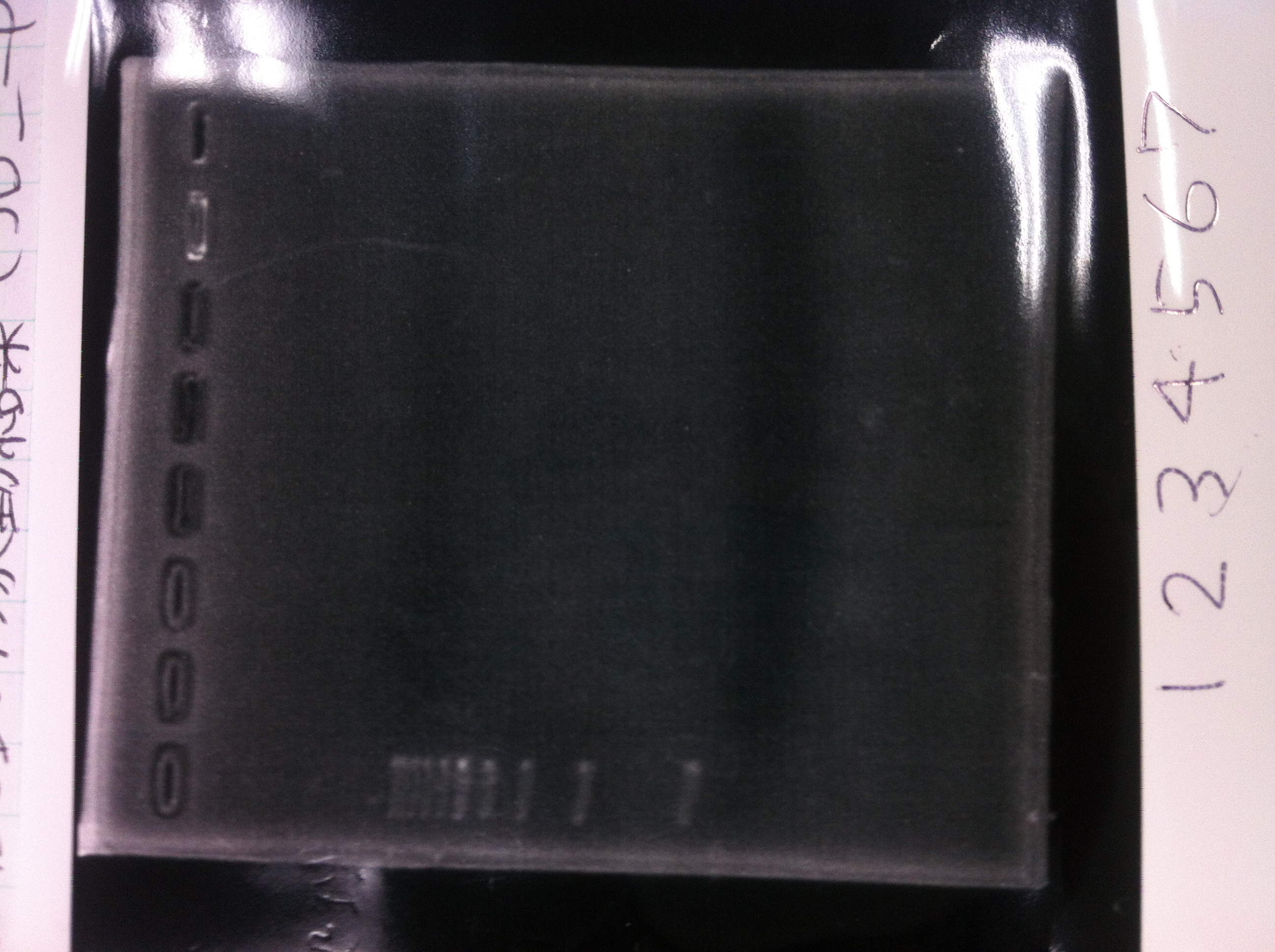
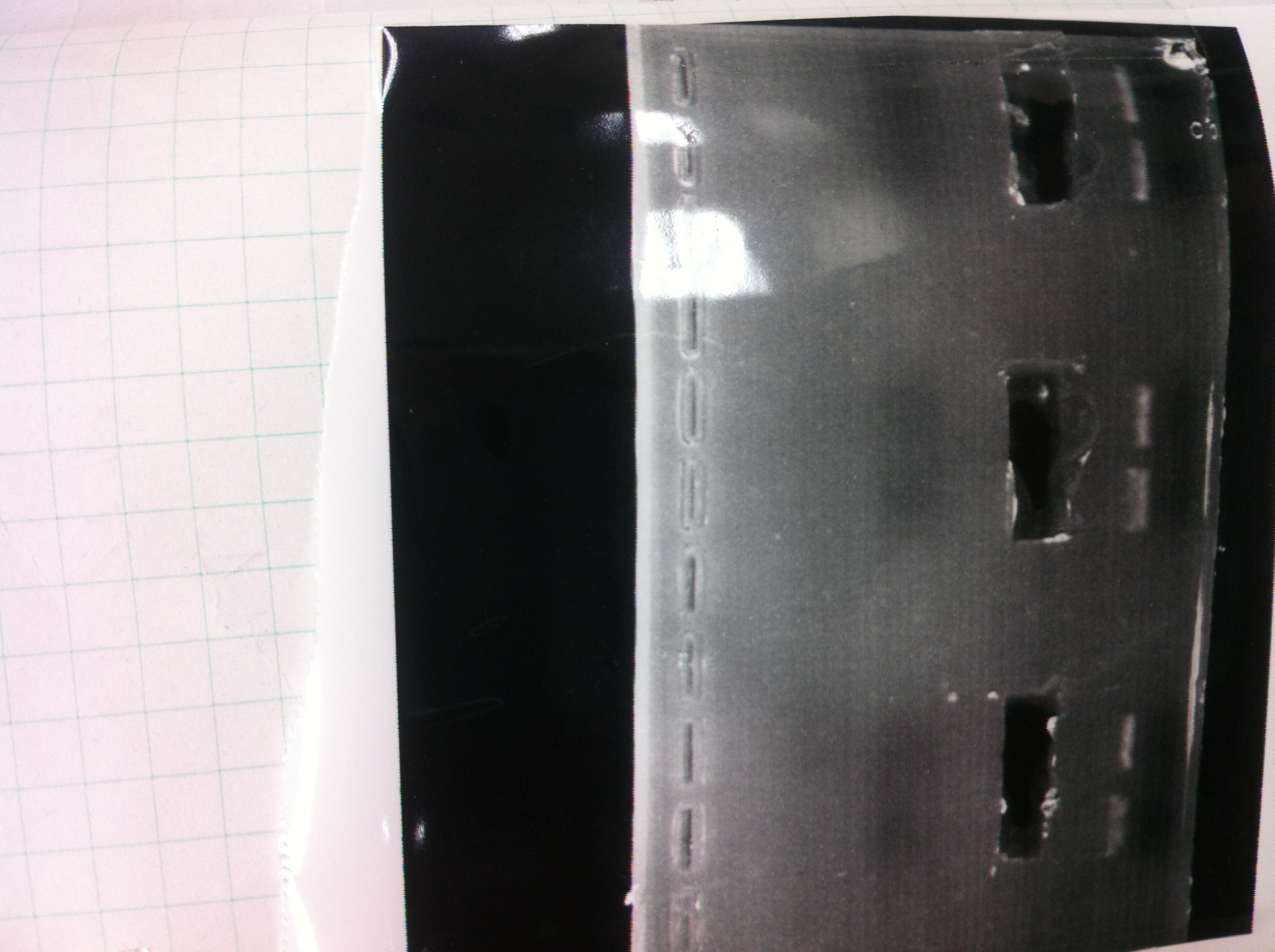
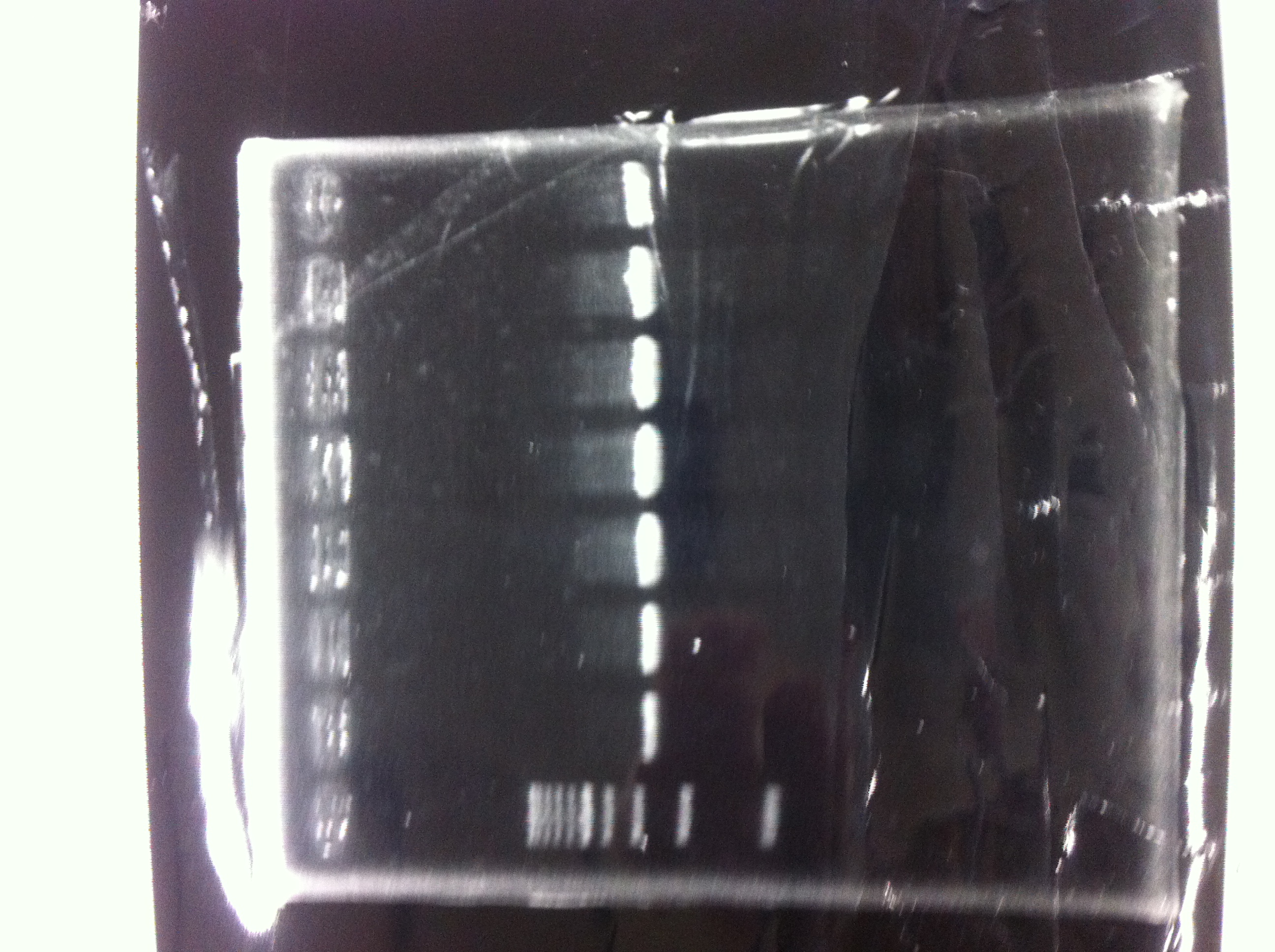
[[File:Electrophoresis021602.JPG|300px|thumb|right]] | [[File:Electrophoresis021602.JPG|300px|thumb|right]] | ||
1. 1kb ladder<br> | 1. 1kb ladder<br> | ||
| Line 176: | Line 184: | ||
We got bands of tatABCD but there were nonspecific amplification products.<br> | We got bands of tatABCD but there were nonspecific amplification products.<br> | ||
We failed amplification of TAMO reductase.<br><br><br><br><br><br><br><br> | We failed amplification of TAMO reductase.<br><br><br><br><br><br><br><br> | ||
| - | + | ====Transformation==== | |
Constitutive promoter (BBa_J23107 , BBA_J23117)<br> | Constitutive promoter (BBa_J23107 , BBA_J23117)<br> | ||
High copy plasmid (pSB1AT3)<br> | High copy plasmid (pSB1AT3)<br> | ||
| Line 183: | Line 191: | ||
|- | |- | ||
|1μL||10μL | |1μL||10μL | ||
| - | |} | + | |} |
| + | |||
==February 17== | ==February 17== | ||
| - | + | ====PCR==== | |
We did PCR to amplify products of PCR that we had done yesterday but we could not amplify tatABCD.<br><br> | We did PCR to amplify products of PCR that we had done yesterday but we could not amplify tatABCD.<br><br> | ||
| - | + | ====Genomic PCR==== | |
{|class="wikitable" | {|class="wikitable" | ||
!Buffer||dNTPs||MgSO4||primer-f||primer-r||genomic DNA||KOD plus||MilliQ||total | !Buffer||dNTPs||MgSO4||primer-f||primer-r||genomic DNA||KOD plus||MilliQ||total | ||
| Line 198: | Line 207: | ||
Extension 68℃, 2.5min<br> | Extension 68℃, 2.5min<br> | ||
(30cycles)<br><br> | (30cycles)<br><br> | ||
| - | + | ====Electrophoresis==== | |
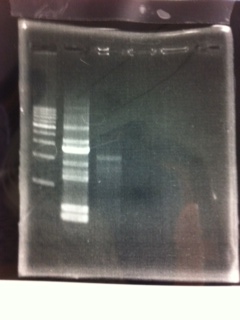
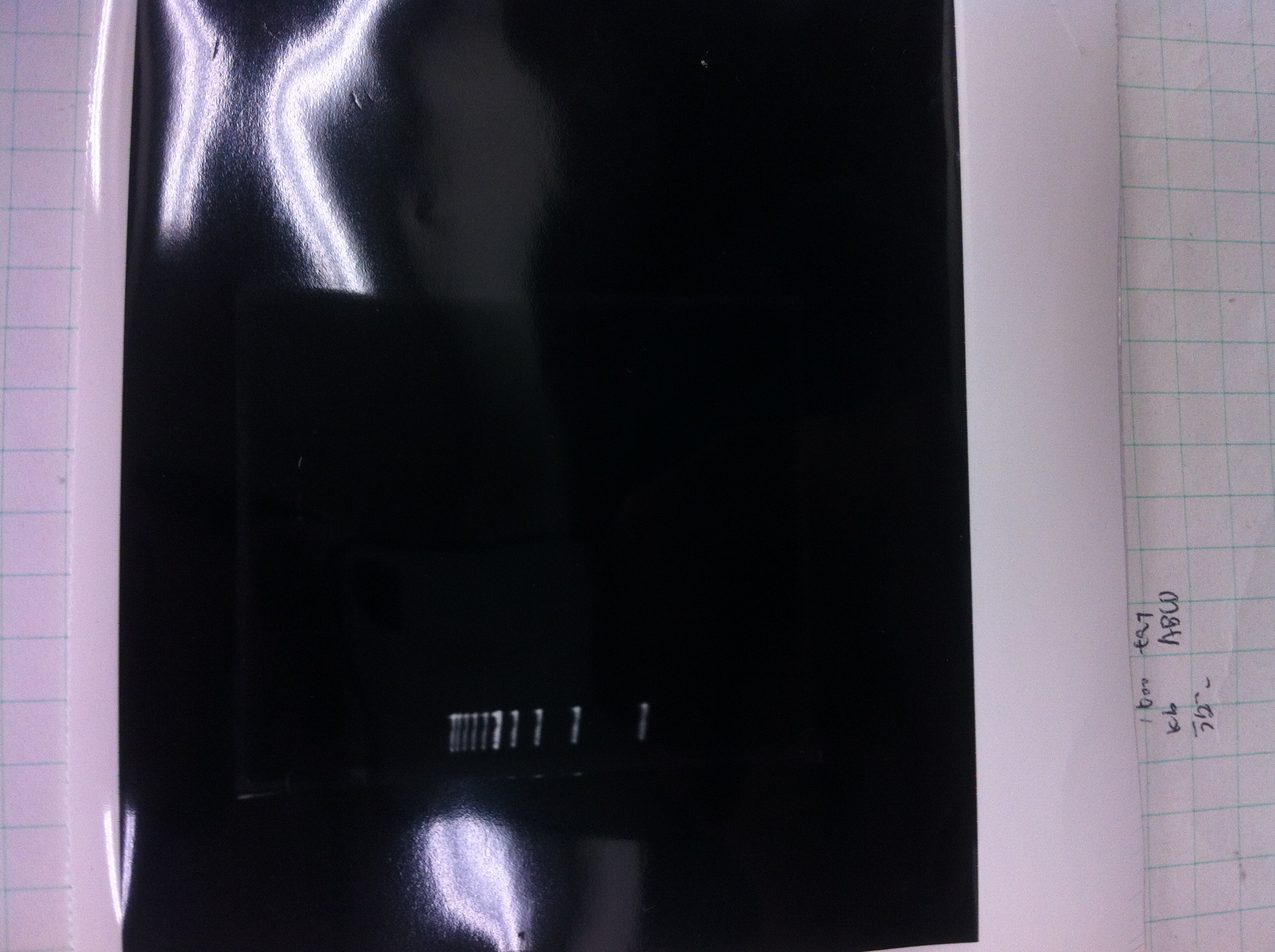
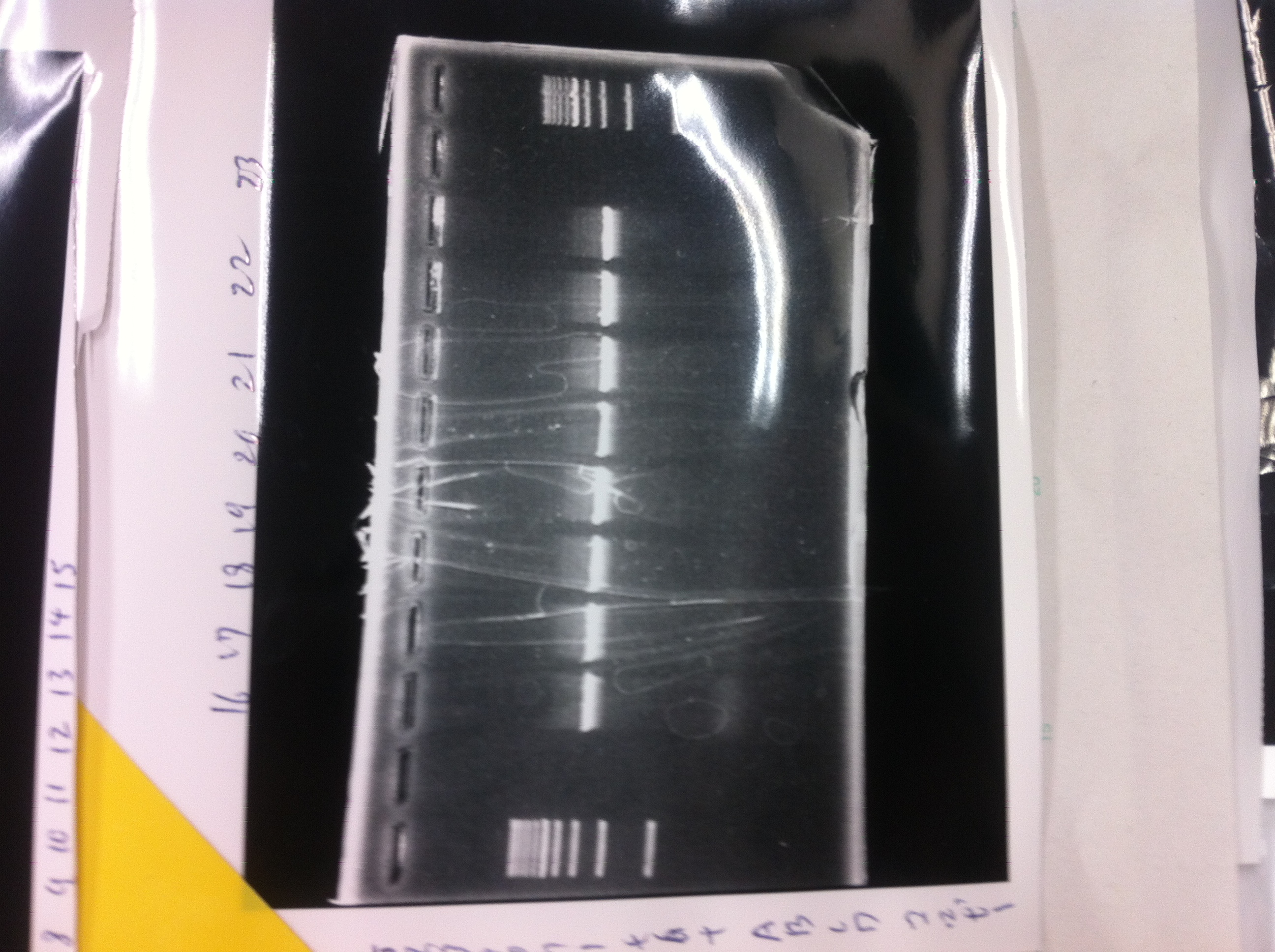
[[File:Electrophoresis021701.JPG|300px|thumb|right]] | [[File:Electrophoresis021701.JPG|300px|thumb|right]] | ||
1. 1kb ladder<br> | 1. 1kb ladder<br> | ||
2. TAMO reductase<br> | 2. TAMO reductase<br> | ||
3. Negative control<br><br> | 3. Negative control<br><br> | ||
| - | + | ====Restriction==== | |
{|class="wikitable" | {|class="wikitable" | ||
!J23100||Spe1||Pst1||Buffer2||BSA||MilliQ||total | !J23100||Spe1||Pst1||Buffer2||BSA||MilliQ||total | ||
| Line 209: | Line 218: | ||
|10||0.5||0.5||3||0.5||15.5||30 | |10||0.5||0.5||3||0.5||15.5||30 | ||
|} | |} | ||
| - | + | ====Genomic PCR==== | |
{|class="wikitable" | {|class="wikitable" | ||
! ||Buffer||dNTPs||MgSO4||primer-f||primer-r||genomic DNA||KOD plus||MilliQ||total | ! ||Buffer||dNTPs||MgSO4||primer-f||primer-r||genomic DNA||KOD plus||MilliQ||total | ||
| Line 219: | Line 228: | ||
|tatABCD||2.5||2.5||2||0.75||0.75||5||0.5||10.5||25 | |tatABCD||2.5||2.5||2||0.75||0.75||5||0.5||10.5||25 | ||
|} | |} | ||
| - | + | ====Electrophoresis==== | |
[[File:Electrophoresis021702.JPG|300px|thumb|right]] | [[File:Electrophoresis021702.JPG|300px|thumb|right]] | ||
1. 1kb ladder <br> | 1. 1kb ladder <br> | ||
| Line 232: | Line 241: | ||
|} | |} | ||
at 37℃ for overnight<br> | at 37℃ for overnight<br> | ||
| - | We checked EcoR1 and Xba1.<br><br><br><br><br><br><br><br> | + | We checked EcoR1 and Xba1.<br><br><br><br><br><br><br><br> |
| + | |||
==February 18== | ==February 18== | ||
| - | + | ====Miniprep==== | |
{|class="wikitable" | {|class="wikitable" | ||
! ||μg/mL||260/280||230/260 | ! ||μg/mL||260/280||230/260 | ||
| Line 251: | Line 261: | ||
|} | |} | ||
diluted to 25 times<br><br> | diluted to 25 times<br><br> | ||
| - | + | ====Competent cell==== | |
We put 3mL of preculture product on yesterday onto 300mL of LB medium | We put 3mL of preculture product on yesterday onto 300mL of LB medium | ||
{|class="wikitable" | {|class="wikitable" | ||
| Line 264: | Line 274: | ||
|13:30||0.502 | |13:30||0.502 | ||
|} | |} | ||
| - | + | ====Transformation==== | |
{|class="wikitable" | {|class="wikitable" | ||
!pSB1AT3-2||competent cell||MilliQ||total | !pSB1AT3-2||competent cell||MilliQ||total | ||
| Line 284: | Line 294: | ||
|10||590 | |10||590 | ||
|} | |} | ||
| - | Transformation efficiency 7.4x10^4 colonys/μg<br> | + | Transformation efficiency 7.4x10^4 colonys/μg<br> |
| + | |||
==February 20== | ==February 20== | ||
| - | + | ====Restriction==== | |
sample 1<br> | sample 1<br> | ||
{|class="wikitable" | {|class="wikitable" | ||
| Line 299: | Line 310: | ||
|10||0.5||0.5||3||0.5||15.5||30 | |10||0.5||0.5||3||0.5||15.5||30 | ||
|} | |} | ||
| - | + | ====Electrophoresis==== | |
*sample1<br> | *sample1<br> | ||
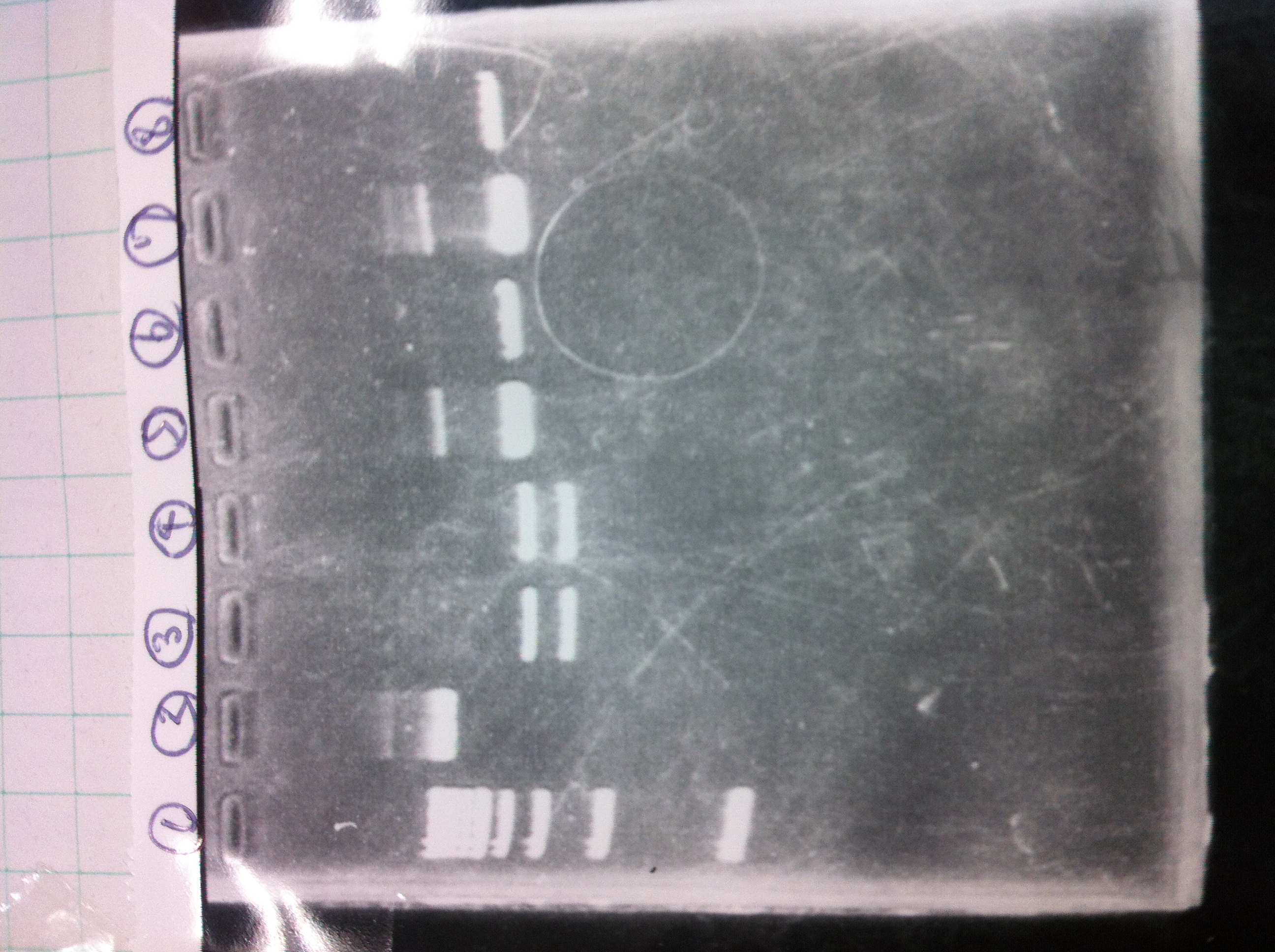
[[File:Electrophoresis022001_.JPG|200px|thumb|right]] | [[File:Electrophoresis022001_.JPG|200px|thumb|right]] | ||
| Line 320: | Line 331: | ||
lane_3 d<br> | lane_3 d<br> | ||
lane_4 1kb ladder<br><br> | lane_4 1kb ladder<br><br> | ||
| - | + | ====PCR==== | |
{|class="wikitable" | {|class="wikitable" | ||
! ||Buffer||dNTPs||MgSO4||primer-f||primer-r||genomic DNA||KOD plus||MilliQ||total | ! ||Buffer||dNTPs||MgSO4||primer-f||primer-r||genomic DNA||KOD plus||MilliQ||total | ||
| Line 367: | Line 378: | ||
lane_4.TAMO<br> | lane_4.TAMO<br> | ||
lane_5.TAMO<br> | lane_5.TAMO<br> | ||
| - | lane_6.1kb ladder<br><br><br><br><br><br> | + | lane_6.1kb ladder<br><br><br><br><br><br> |
| + | |||
==February 21== | ==February 21== | ||
| - | + | ====PCR (Advantage HF protocol)==== | |
{|class="wikitable" | {|class="wikitable" | ||
! ||buffer||dNTPs||primer-f||primer-r||gDNA||PCR products||DW||polymerase||total | ! ||buffer||dNTPs||primer-f||primer-r||gDNA||PCR products||DW||polymerase||total | ||
| Line 382: | Line 394: | ||
Extension 68℃ 3min<br> | Extension 68℃ 3min<br> | ||
→25cycles<br><br> | →25cycles<br><br> | ||
| - | + | ====Electrophoresis==== | |
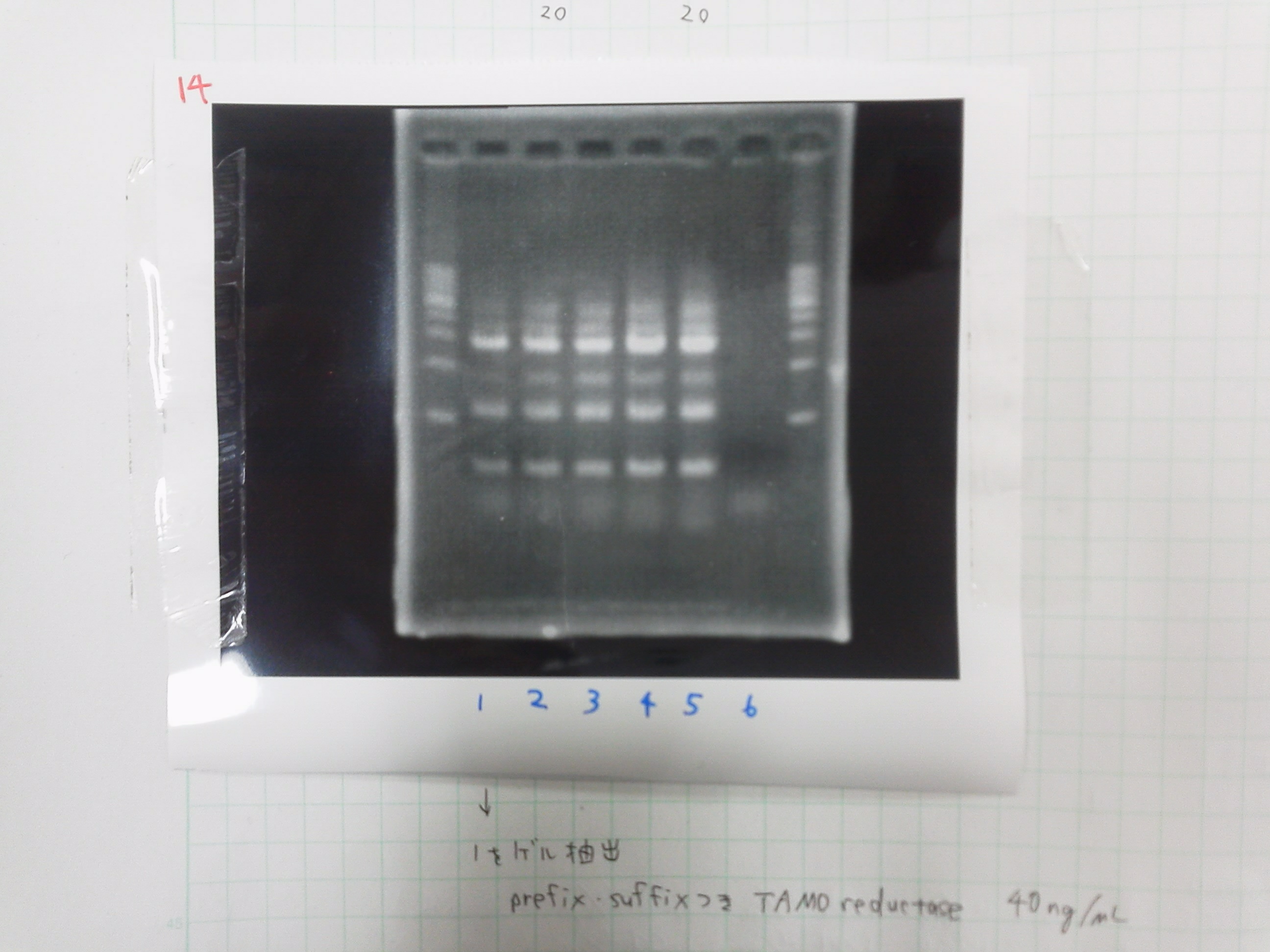
[[File:Electrophoresis0221.JPG|250px|thumb|right]] | [[File:Electrophoresis0221.JPG|250px|thumb|right]] | ||
1. 1kb ladder 2μL<br> | 1. 1kb ladder 2μL<br> | ||
| Line 390: | Line 402: | ||
'''Liquid culture'''<br> | '''Liquid culture'''<br> | ||
pSB3C5-1,2<br> | pSB3C5-1,2<br> | ||
| - | pSB4K5-1,2 | + | pSB4K5-1,2 |
| + | |||
==February 22== | ==February 22== | ||
| - | + | ====Gel extraction==== | |
lane 1 of the gel 45.0μg/mL<br><br> | lane 1 of the gel 45.0μg/mL<br><br> | ||
| - | + | ====PCR purification==== | |
product 38.2μg/mL <br><br> | product 38.2μg/mL <br><br> | ||
| - | + | ====PCR==== | |
TMAO reductase<br> | TMAO reductase<br> | ||
{|class="wikitable" | {|class="wikitable" | ||
| Line 414: | Line 427: | ||
68℃, 3min<br> | 68℃, 3min<br> | ||
→25cycles<br><br> | →25cycles<br><br> | ||
| - | + | ====Electrophoresis==== | |
[[File:Electrophoresis022201.JPG|250px|thumb|right]] | [[File:Electrophoresis022201.JPG|250px|thumb|right]] | ||
1. 1kb ladder<br> | 1. 1kb ladder<br> | ||
| Line 425: | Line 438: | ||
8. constructive promoter Pst1<br> | 8. constructive promoter Pst1<br> | ||
9. 1kb ladder<br> | 9. 1kb ladder<br> | ||
| - | + | ====PCR==== | |
{|class="wikitable" | {|class="wikitable" | ||
! ||Buffer||dNTPs||MgSO4||prefix primer-f||suffix primer-r||product of PCR purification(1ng/μL)||KOD plus||MilliQ||total | ! ||Buffer||dNTPs||MgSO4||prefix primer-f||suffix primer-r||product of PCR purification(1ng/μL)||KOD plus||MilliQ||total | ||
| Line 446: | Line 459: | ||
68℃, 3min<br> | 68℃, 3min<br> | ||
→25cycles<br><br> | →25cycles<br><br> | ||
| - | + | ====Electrophoresis==== | |
[[File:Electrophoresis022202.JPG|300px|thumb|right]]<br><br><br><br><br><br><br><br><br><br><br><br> | [[File:Electrophoresis022202.JPG|300px|thumb|right]]<br><br><br><br><br><br><br><br><br><br><br><br> | ||
| - | + | ====Checking Dpn1==== | |
{|class="wikitable" | {|class="wikitable" | ||
| - | !Buffer|| | + | !Buffer||LacP(28.7ng/μL)||Dpn1||MilliQ||total |
|- | |- | ||
|2||10||0.5||7.5||20 | |2||10||0.5||7.5||20 | ||
|- | |- | ||
|2||10||0||5||17 | |2||10||0||5||17 | ||
| - | |} | + | |} |
| + | |||
==February 23== | ==February 23== | ||
| - | + | ====Colony PCR==== | |
tatABCD(2 samples)<br> | tatABCD(2 samples)<br> | ||
{|class="wikitable" | {|class="wikitable" | ||
| Line 469: | Line 483: | ||
Extension 68℃ 3min<br> | Extension 68℃ 3min<br> | ||
→25cycles<br><br> | →25cycles<br><br> | ||
| - | + | ====Electrophoresis==== | |
[[File:Electrophoresis022301.JPG|300px|thumb|right]] | [[File:Electrophoresis022301.JPG|300px|thumb|right]] | ||
1. 1kb ladder 2μL<br> | 1. 1kb ladder 2μL<br> | ||
| Line 475: | Line 489: | ||
3. tatABCD 2 5μL + 6×Loading Buffer 1μL<br> | 3. tatABCD 2 5μL + 6×Loading Buffer 1μL<br> | ||
4. 1kb ladder 2μL<br><br> | 4. 1kb ladder 2μL<br><br> | ||
| - | + | ====Miniprep==== | |
pSB4K5 and pSB3C5<br> | pSB4K5 and pSB3C5<br> | ||
deluted it to 25 times and then measured it<br> | deluted it to 25 times and then measured it<br> | ||
| Line 482: | Line 496: | ||
pSB3C5-3 : 3.6 μg/ml 1.57(260/280) 3.00(260/230) <br> | pSB3C5-3 : 3.6 μg/ml 1.57(260/280) 3.00(260/230) <br> | ||
pSB3C5-4 : 1.3 μg/ml 1.44(260/280) 1.04(260/230) <br><br> | pSB3C5-4 : 1.3 μg/ml 1.44(260/280) 1.04(260/230) <br><br> | ||
| - | + | ====Liquid culture==== | |
pSB3C5-3,4<br><br> | pSB3C5-3,4<br><br> | ||
| - | + | ====Electrophoresis==== | |
[[File:Electrophoresis022302.JPG|300px|thumb|right]] | [[File:Electrophoresis022302.JPG|300px|thumb|right]] | ||
1. 1kb ladder 2μL<br> | 1. 1kb ladder 2μL<br> | ||
2. pSB3C5-3 5μL, 6×loading dye 1μL<br> | 2. pSB3C5-3 5μL, 6×loading dye 1μL<br> | ||
3. pSB3C5-4 5μL, 6×loading dye 1μL<br> | 3. pSB3C5-4 5μL, 6×loading dye 1μL<br> | ||
| - | 4. 1kb ladder 2μL<br><br><br><br><br><br><br><br> | + | 4. 1kb ladder 2μL<br><br><br><br><br><br><br><br> |
| + | |||
==February 27== | ==February 27== | ||
| - | + | ====Test of Dpn1==== | |
{| class="wikitable" | {| class="wikitable" | ||
!Buffer2||GFP2||BSA||MilliQ||Dpn1 | !Buffer2||GFP2||BSA||MilliQ||Dpn1 | ||
| Line 497: | Line 512: | ||
|3||3||0.3||23||1 | |3||3||0.3||23||1 | ||
|} | |} | ||
| - | + | ====Colony PCR==== | |
{| class="wikitable" | {| class="wikitable" | ||
! ||buffer||dNTPs||MgSO4||Primer-f||Primer-r||MilliQ||KOD Plus||total | ! ||buffer||dNTPs||MgSO4||Primer-f||Primer-r||MilliQ||KOD Plus||total | ||
| Line 510: | Line 525: | ||
Extension 68℃,3min<br> | Extension 68℃,3min<br> | ||
→25cycles<br><br> | →25cycles<br><br> | ||
| - | + | ====Electrophoresis==== | |
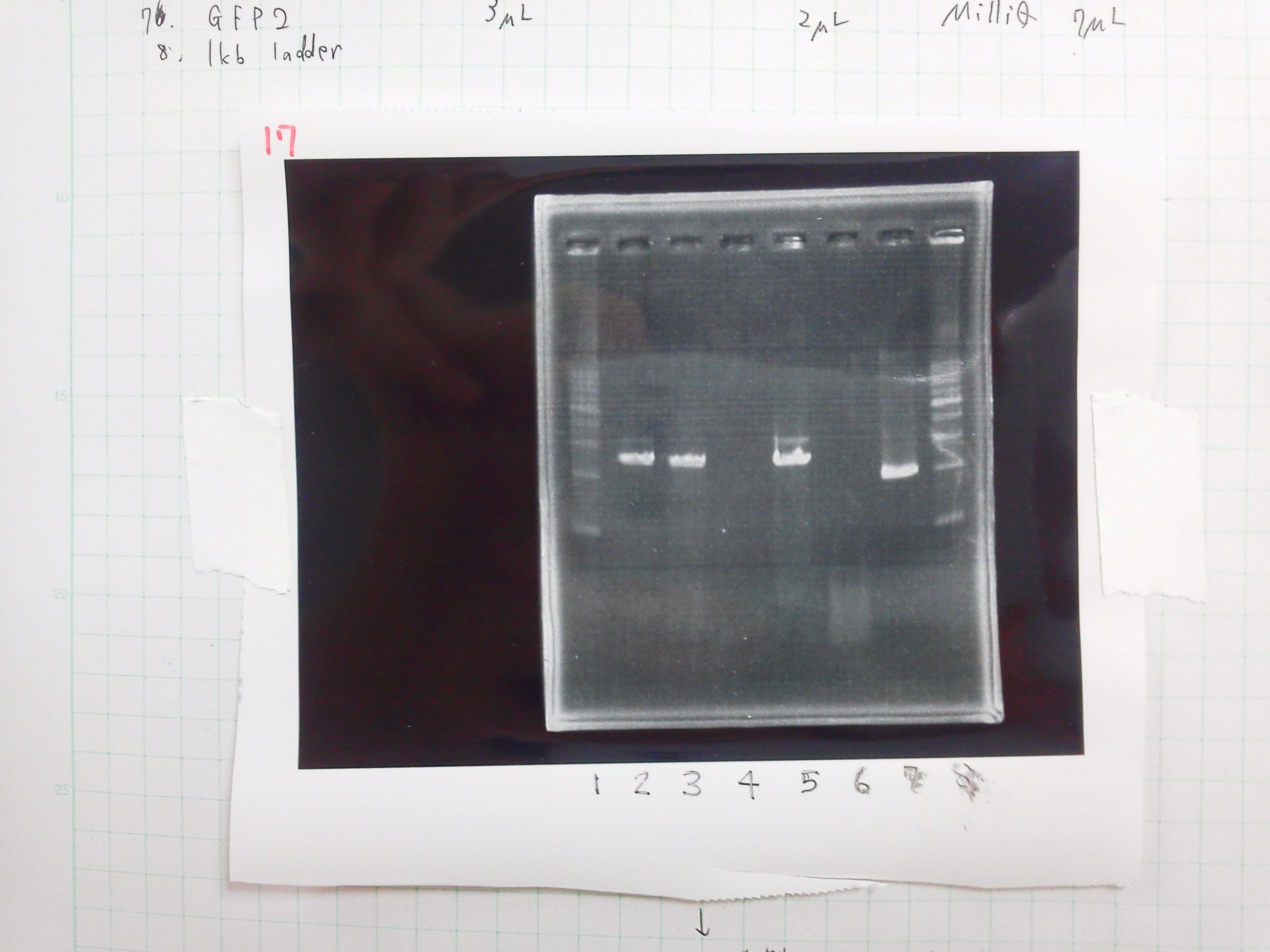
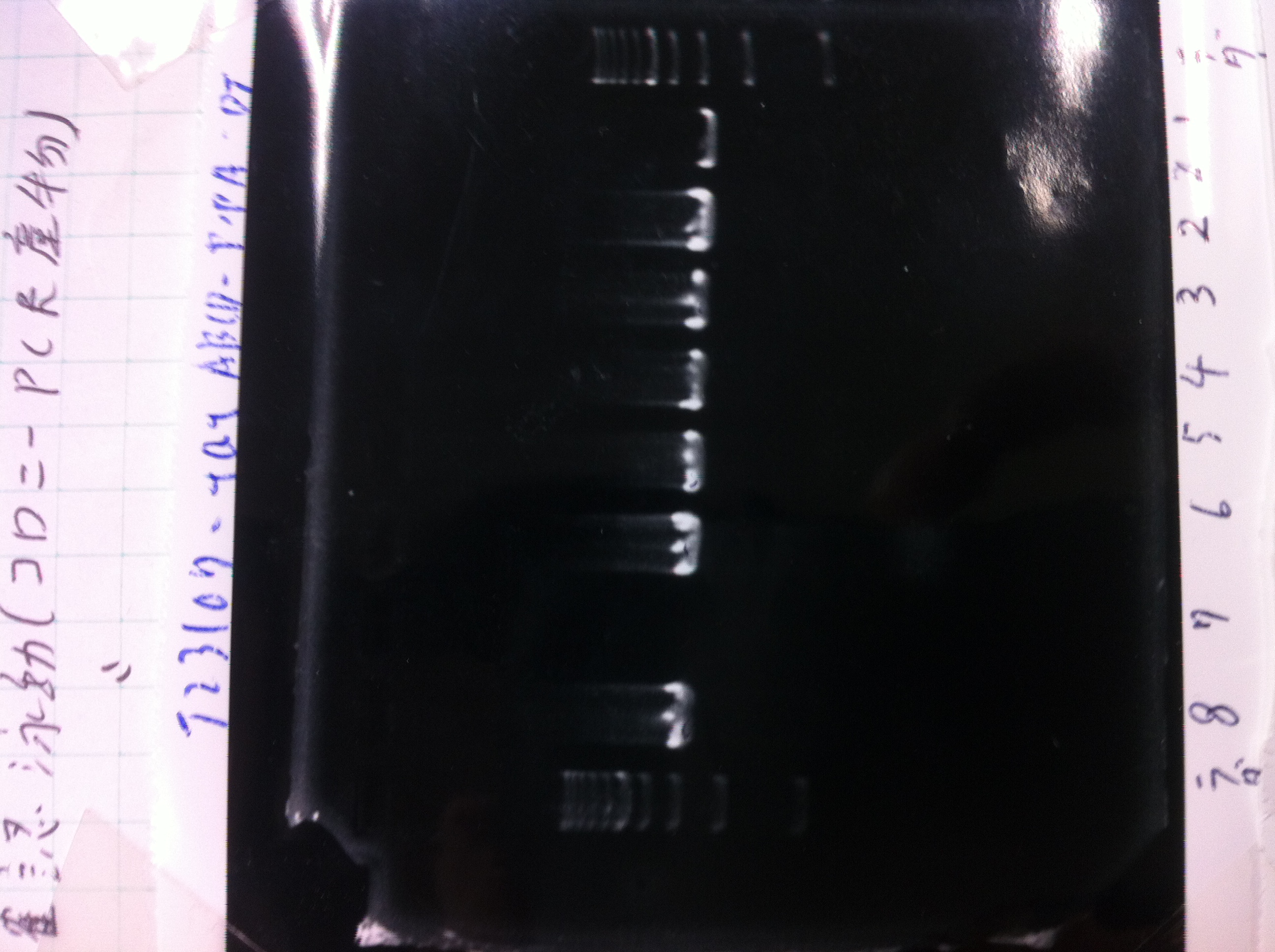
[[File:Electrophoresis0227.JPG|200px|thumb|right]] | [[File:Electrophoresis0227.JPG|200px|thumb|right]] | ||
{| class="wikitable" | {| class="wikitable" | ||
| Line 531: | Line 546: | ||
|8.1kb ladder||2||0||0 | |8.1kb ladder||2||0||0 | ||
|} | |} | ||
| - | + | ====Results of liquid culture==== | |
We measure this after dilute it to 10 times.<br> | We measure this after dilute it to 10 times.<br> | ||
{|class="wikitable" | {|class="wikitable" | ||
| Line 538: | Line 553: | ||
|8.5[µg/ml]||-1.8||-17.9||-18.2 | |8.5[µg/ml]||-1.8||-17.9||-18.2 | ||
|} | |} | ||
| - | + | ====PCR==== | |
{| class="wikitable" | {| class="wikitable" | ||
! ||buffer||dNTPs||MgSO4||Primer-f(prefix)||Primer-r(suffix)||PCR purification product(1ng/µL)||MilliQ||KOD Plus||total | ! ||buffer||dNTPs||MgSO4||Primer-f(prefix)||Primer-r(suffix)||PCR purification product(1ng/µL)||MilliQ||KOD Plus||total | ||
| Line 551: | Line 566: | ||
Annealing 59℃,30sec<br> | Annealing 59℃,30sec<br> | ||
Extension 68℃,3min<br> | Extension 68℃,3min<br> | ||
| - | →25cycles<br><br> | + | →25cycles<br><br> |
| + | |||
==February 28== | ==February 28== | ||
| - | + | ====Electrophoresis==== | |
[[File:Electrophoresis022801.JPG|250px|thumb|right]] | [[File:Electrophoresis022801.JPG|250px|thumb|right]] | ||
1. 1kb ladder<br> | 1. 1kb ladder<br> | ||
| Line 559: | Line 575: | ||
3. PCR2<br><br> | 3. PCR2<br><br> | ||
| - | + | ====Restriction==== | |
{| class="wikitable" | {| class="wikitable" | ||
!Buffer2||plasmid(?)||enzyme||MilliQ||total | !Buffer2||plasmid(?)||enzyme||MilliQ||total | ||
| Line 566: | Line 582: | ||
|} | |} | ||
incubate 1 hour at 37℃<br><br> | incubate 1 hour at 37℃<br><br> | ||
| - | + | ====Electrophoresis==== | |
[[File:Electrophoresis022802.JPG|250px|thumb|right]] | [[File:Electrophoresis022802.JPG|250px|thumb|right]] | ||
[[File:Electrophoresis022803.JPG|250px|thumb|right]] | [[File:Electrophoresis022803.JPG|250px|thumb|right]] | ||
| Line 577: | Line 593: | ||
7. Pst1<br> | 7. Pst1<br> | ||
8. 1kb ladder<br><br> | 8. 1kb ladder<br><br> | ||
| - | + | ====PCR and Electrophoresis==== | |
{| class="wikitable" | {| class="wikitable" | ||
!Quick Taq Dye Mix||primer-f||primer-r||template||MilliQ||total | !Quick Taq Dye Mix||primer-f||primer-r||template||MilliQ||total | ||
| Line 588: | Line 604: | ||
Extension 68℃,3min<br> | Extension 68℃,3min<br> | ||
→25cycles<br><br> | →25cycles<br><br> | ||
| - | + | ====Restriction==== | |
{| class="wikitable" | {| class="wikitable" | ||
!BufferH||tatABCD||EcoR1||Spe1||MilliQ||total | !BufferH||tatABCD||EcoR1||Spe1||MilliQ||total | ||
| Line 594: | Line 610: | ||
|2||5||0.2||0.2||12.6||20 | |2||5||0.2||0.2||12.6||20 | ||
|} | |} | ||
| - | + | ====PCR purification==== | |
We eluted the product for 30µL MilliQ<br><br> | We eluted the product for 30µL MilliQ<br><br> | ||
| - | + | ====Ligation==== | |
{| class="wikitable" | {| class="wikitable" | ||
!Insert(tatABCD)||Vector(pSB1C3)||Ligation High||total | !Insert(tatABCD)||Vector(pSB1C3)||Ligation High||total | ||
| Line 602: | Line 618: | ||
|10||1||5||16 | |10||1||5||16 | ||
|} | |} | ||
| - | 4℃, overnight | + | 4℃, overnight |
| + | |||
==February 29== | ==February 29== | ||
| - | + | ====Transformation==== | |
{|class="wikitable" | {|class="wikitable" | ||
!tatABCD||competent cell||total | !tatABCD||competent cell||total | ||
| Line 610: | Line 627: | ||
|1||10||11 | |1||10||11 | ||
|} | |} | ||
| - | + | ====Checking Restriction enzyme==== | |
{|class="wikitable" | {|class="wikitable" | ||
!plasmid seems to be 1-18C promoter||Enzyme||Buffer||MilliQ||total | !plasmid seems to be 1-18C promoter||Enzyme||Buffer||MilliQ||total | ||
| Line 616: | Line 633: | ||
|2||0.2||2||15.8||20 | |2||0.2||2||15.8||20 | ||
|} | |} | ||
| - | + | ====Checking tatABCD==== | |
{| class="wikitable" | {| class="wikitable" | ||
!tatABCD||Hind3||Buffer||MilliQ||total | !tatABCD||Hind3||Buffer||MilliQ||total | ||
| Line 622: | Line 639: | ||
|5||0.2||2||12.8||20 | |5||0.2||2||12.8||20 | ||
|} | |} | ||
| - | + | ====PCR==== | |
{| class="wikitable" | {| class="wikitable" | ||
! ||buffer||dNTPs||MgSO4||Primer-f||Primer-r||ColE1(6.5ng/µL) / TMAO||MilliQ||KOD Plus Neo||total | ! ||buffer||dNTPs||MgSO4||Primer-f||Primer-r||ColE1(6.5ng/µL) / TMAO||MilliQ||KOD Plus Neo||total | ||
| Line 635: | Line 652: | ||
Extension 68℃,3min<br> | Extension 68℃,3min<br> | ||
→30cycles<br><br> | →30cycles<br><br> | ||
| - | + | ====Electrophoresis==== | |
*evernoteに写真なし!至急求む | *evernoteに写真なし!至急求む | ||
1. 1kb ladder<br> | 1. 1kb ladder<br> | ||
| Line 643: | Line 660: | ||
5. tatABCD (Quick Taq)<br> | 5. tatABCD (Quick Taq)<br> | ||
6. tatABCD (Hind3)<br> | 6. tatABCD (Hind3)<br> | ||
| - | 7. 1kb ladder<br> | + | 7. 1kb ladder<br> |
| - | + | ||
==March 1== | ==March 1== | ||
| - | + | ====PCR==== | |
*TMAO<br> | *TMAO<br> | ||
Template is gDNA and product of colony PCR gel extraction<br> | Template is gDNA and product of colony PCR gel extraction<br> | ||
| Line 672: | Line 689: | ||
68℃, 1min<br> | 68℃, 1min<br> | ||
→20cycles<br><br> | →20cycles<br><br> | ||
| - | + | ====Electrophoresis==== | |
| - | + | [[File:Electrophoresis030101.JPG|250px|thumb|right]] | |
| - | + | [[File:Electrophoresis030102.JPG|250px|thumb|right]] | |
1. 1kb ladder<br> | 1. 1kb ladder<br> | ||
2. TMAO1 (gDNA)<br> | 2. TMAO1 (gDNA)<br> | ||
3. TMAO2 (product of gel extraction)<br> | 3. TMAO2 (product of gel extraction)<br> | ||
4. Kil<br><br> | 4. Kil<br><br> | ||
| - | + | ====PCR==== | |
{| class="wikitable" | {| class="wikitable" | ||
!Buffer||dNTPs||MgSO4||primer-f||primer-r||Product of Purification||KOD Plus Neo||MilliQ||total | !Buffer||dNTPs||MgSO4||primer-f||primer-r||Product of Purification||KOD Plus Neo||MilliQ||total | ||
| Line 691: | Line 708: | ||
20cycles<br> | 20cycles<br> | ||
→Purification 230ng/µL<br><br> | →Purification 230ng/µL<br><br> | ||
| - | + | ====Restriction==== | |
{| class="wikitable" | {| class="wikitable" | ||
!Kil||EcoR1||Spe1||BufferH||MilliQ||total | !Kil||EcoR1||Spe1||BufferH||MilliQ||total | ||
| Line 698: | Line 715: | ||
|} | |} | ||
incubate at 37℃, for 1.5 hours<br><br> | incubate at 37℃, for 1.5 hours<br><br> | ||
| - | + | ====PCR Purification==== | |
| - | + | ====Ligation==== | |
{| class="wikitable" | {| class="wikitable" | ||
!Kil||pSB1C3||Ligation High||total | !Kil||pSB1C3||Ligation High||total | ||
| Line 705: | Line 722: | ||
|5||1||3||9 | |5||1||3||9 | ||
|} | |} | ||
| - | at 4℃, for overnight | + | at 4℃, for overnight |
| + | |||
==March 2== | ==March 2== | ||
| - | + | ====Ligation==== | |
{| class="wikitable" | {| class="wikitable" | ||
!Kil||pSB1C3||Ligation High Ver.2||total | !Kil||pSB1C3||Ligation High Ver.2||total | ||
| Line 719: | Line 737: | ||
|} | |} | ||
at 16℃ for 1 hour<br><br> | at 16℃ for 1 hour<br><br> | ||
| - | + | ====Transformation==== | |
{| class="wikitable" | {| class="wikitable" | ||
!Kil||Kil(3/1,Ligation)||tatABCD||competet cell||total | !Kil||Kil(3/1,Ligation)||tatABCD||competet cell||total | ||
| Line 729: | Line 747: | ||
|0||0||1||10||11 | |0||0||1||10||11 | ||
|} | |} | ||
| - | + | ====PCR==== | |
{| class="wikitable" | {| class="wikitable" | ||
!Quick Taq||primer-r||primer-f||template||MilliQ||total | !Quick Taq||primer-r||primer-f||template||MilliQ||total | ||
| Line 735: | Line 753: | ||
|25||1||1||0.5||22.5||50 | |25||1||1||0.5||22.5||50 | ||
|} | |} | ||
| - | + | ====Electrophoresis==== | |
[[File:Electrophoresis030227.JPG|250px|thumb|right]] | [[File:Electrophoresis030227.JPG|250px|thumb|right]] | ||
<br><br> | <br><br> | ||
| - | + | ====Restriction==== | |
{| class="wikitable" | {| class="wikitable" | ||
!pSB3C5-5||EcoR1||Pst1||BufferH||BSA||MilliQ||total | !pSB3C5-5||EcoR1||Pst1||BufferH||BSA||MilliQ||total | ||
| Line 745: | Line 763: | ||
|} | |} | ||
at 37℃, for 2 hour<br><br> | at 37℃, for 2 hour<br><br> | ||
| - | + | ====Electrophoresis==== | |
[[File:Electrophoresis030225.JPG|250px|thumb|right]] | [[File:Electrophoresis030225.JPG|250px|thumb|right]] | ||
1. 1kb ladder 2µL<br> | 1. 1kb ladder 2µL<br> | ||
| Line 751: | Line 769: | ||
・product of gel extraction(about 2700bp)<br> | ・product of gel extraction(about 2700bp)<br> | ||
-30.9µg/mL<br><br> | -30.9µg/mL<br><br> | ||
| - | + | ====Restriction==== | |
| - | GFP1,2,3 | + | GFP1 ,2 ,3 |
{| class="wikitable" | {| class="wikitable" | ||
!GFP||EcoR1||Pst1||Buffer||BSA||MilliQ||total | !GFP||EcoR1||Pst1||Buffer||BSA||MilliQ||total | ||
| Line 759: | Line 777: | ||
|} | |} | ||
at 37℃, for 2.5 hours<br><br> | at 37℃, for 2.5 hours<br><br> | ||
| - | + | ====Restriction==== | |
{| class="wikitable" | {| class="wikitable" | ||
!DT||EcoR1||Xba1||BufferM||BSA||MilliQ||total | !DT||EcoR1||Xba1||BufferM||BSA||MilliQ||total | ||
| Line 774: | Line 792: | ||
*DT3→PCR Purification<br> | *DT3→PCR Purification<br> | ||
*Promoter→Gel Extraction<br><br> | *Promoter→Gel Extraction<br><br> | ||
| - | + | ====Checking TMAO==== | |
{| class="wikitable" | {| class="wikitable" | ||
!something seems to be TMAO||Buffer2||EcoR1||MilliQ||total | !something seems to be TMAO||Buffer2||EcoR1||MilliQ||total | ||
| Line 780: | Line 798: | ||
|} | |} | ||
at 37℃, for 1 hour<br><br> | at 37℃, for 1 hour<br><br> | ||
| - | + | ====Electrophoresis==== | |
[[File:Electrophoresis030226.JPG|250px|thumb|right]] | [[File:Electrophoresis030226.JPG|250px|thumb|right]] | ||
1. 1kb ladder<br> | 1. 1kb ladder<br> | ||
| Line 800: | Line 818: | ||
|25||1||1|||23||50 | |25||1||1|||23||50 | ||
|} | |} | ||
| - | + | ====Electrophoresis==== | |
[[File:Electrophoresis030228.JPG|250px|thumb|right]] | [[File:Electrophoresis030228.JPG|250px|thumb|right]] | ||
| - | <br><br><br><br><br><br><br><br><br><br><br><br> | + | <br><br><br><br><br><br><br><br><br><br><br><br> |
| + | |||
==March 3== | ==March 3== | ||
====PCR==== | ====PCR==== | ||
| Line 907: | Line 926: | ||
====Restriction==== | ====Restriction==== | ||
{| class="wikitable" | {| class="wikitable" | ||
| - | ! || | + | ! ||LacP||pSB3C5||EcoR1||Pst1||BufferH||BSA||MilliQ||total |
|- | |- | ||
|1||20||0||0.5||0.5||3||0.5||5.5||30 | |1||20||0||0.5||0.5||3||0.5||5.5||30 | ||
| Line 925: | Line 944: | ||
====Transformation==== | ====Transformation==== | ||
{| class="wikitable" | {| class="wikitable" | ||
| - | ! | + | !LacP+pSB3C5||competent cell||total |
|- | |- | ||
|1||10||11 | |1||10||11 | ||
| Line 1,069: | Line 1,088: | ||
*pSB4K5→36.6ng/µL×1µL=36.6ng=86fmol<br><br> | *pSB4K5→36.6ng/µL×1µL=36.6ng=86fmol<br><br> | ||
====Liquid culture==== | ====Liquid culture==== | ||
| - | + | LacP + pSB3C5 -1, 2<br><br> | |
====Transformation==== | ====Transformation==== | ||
{| class="wikitable" | {| class="wikitable" | ||
| Line 1,088: | Line 1,107: | ||
→purification : 75.0μg/mL (1.11 260/280 , 0.81 260/230)<br><br> | →purification : 75.0μg/mL (1.11 260/280 , 0.81 260/230)<br><br> | ||
====Miniprep==== | ====Miniprep==== | ||
| - | + | LacP + pSB3C5 1 80.5μg/mL (1.78 260/280 , 2.00 260/230)<br> | |
| - | + | LacP + pSB3C5 2 107.2μg/mL (1.83 260/280 , 1.90 260/230)<br><br> | |
====Colony PCR==== | ====Colony PCR==== | ||
{| class="wikitable" | {| class="wikitable" | ||
| Line 1,154: | Line 1,173: | ||
====Ligation==== | ====Ligation==== | ||
{| class="wikitable" | {| class="wikitable" | ||
| - | !torA|| | + | !torA||LacP-pSB3C5||Ligation High Ver.2||total |
|- | |- | ||
|4||2||3||9 | |4||2||3||9 | ||
|} | |} | ||
torA : 929fmol<br> | torA : 929fmol<br> | ||
| - | + | LacP-pSB3C5 : 284fmol<br> | |
for overnight at 4℃ | for overnight at 4℃ | ||
==March 11== | ==March 11== | ||
| Line 1,188: | Line 1,207: | ||
====Transformation==== | ====Transformation==== | ||
{| class="wikitable" | {| class="wikitable" | ||
| - | !DT(1ng/μL)||DT(0.1ng/μL)||Kil|| | + | !DT(1ng/μL)||DT(0.1ng/μL)||Kil||LacP-torA||MilliQ||competent cell||total |
|- | |- | ||
|1||0||0||0||0||20||21 | |1||0||0||0||0||20||21 | ||
| Line 1,243: | Line 1,262: | ||
*pSB1C3 : 0.068pmol<br><br> | *pSB1C3 : 0.068pmol<br><br> | ||
====Liquid culture==== | ====Liquid culture==== | ||
| - | We cultured | + | We cultured LacP-pSB3C5 1,2,3,4,5 (CP tolerance)on culture with Amp.<br> |
→Only 4 which did not be cultured succeeded. | →Only 4 which did not be cultured succeeded. | ||
==March 15== | ==March 15== | ||
====Liquid culture==== | ====Liquid culture==== | ||
| - | We cultured | + | We cultured LacP-pSB3C5 6,7,8,9,10 on culture with ampicillin.<br> |
→6,8,9,10 were succeeded.<br><br> | →6,8,9,10 were succeeded.<br><br> | ||
====Restriction==== | ====Restriction==== | ||
| Line 1,540: | Line 1,559: | ||
pspA 11.5ng/µL.<br><br> | pspA 11.5ng/µL.<br><br> | ||
====Miniprep==== | ====Miniprep==== | ||
| - | + | LacP+pSB3C5-8 77.9µg/mL<br> | |
| - | + | LacP+pSB3C5-10 69.0µg/mL<br> | |
Kil+DT-4 58.0µg/mL<br> | Kil+DT-4 58.0µg/mL<br> | ||
Kil+DT-7 15.2µg/mL<br><br> | Kil+DT-7 15.2µg/mL<br><br> | ||
====Restriction==== | ====Restriction==== | ||
{| class="wikitable" | {| class="wikitable" | ||
| - | ! | + | !LacP+pSB3C5-8||Spe1||Pst1||Buffer2||BSA||MilliQ||total |
|- | |- | ||
|20||0.5||0.5||3||0.5||5.5||30 | |20||0.5||0.5||3||0.5||5.5||30 | ||
| Line 1,558: | Line 1,577: | ||
And then we did Gel extraction.<br><br> | And then we did Gel extraction.<br><br> | ||
====Gel Extraction==== | ====Gel Extraction==== | ||
| - | + | LacP+pSB3C5-8 40.4µg/mL<br> | |
Kil+DT-4 26.8µg/mL<br> | Kil+DT-4 26.8µg/mL<br> | ||
| Line 1,585: | Line 1,604: | ||
====Ligation==== | ====Ligation==== | ||
{| class="wikitable" | {| class="wikitable" | ||
| - | ! | + | !LacP (pSB3C5)||torA-GFP-DT||Ligation High Ver.2||total |
|- | |- | ||
|1||5||3||9 | |1||5||3||9 | ||
|} | |} | ||
| - | * | + | *LacP : 22fmol<br> |
*torA-GFP-DT : 197fmol<br> | *torA-GFP-DT : 197fmol<br> | ||
{| class="wikitable" | {| class="wikitable" | ||
| Line 1,612: | Line 1,631: | ||
====Transformation==== | ====Transformation==== | ||
{| class="wikitable" | {| class="wikitable" | ||
| - | ! | + | !LacP-Kil-DT||competent cell||total |
|- | |- | ||
|1||10||11 | |1||10||11 | ||
| Line 1,634: | Line 1,653: | ||
|25||1||1||23||50 | |25||1||1||23||50 | ||
|} | |} | ||
| - | + | LacP-torA-GFP-DT 1, 3, 5, 6, 8, 9 was successful.<br> | |
pspA-DT was failed.<br> | pspA-DT was failed.<br> | ||
E.coli that had pspA(pSB1C3) did not make any colony.<br> | E.coli that had pspA(pSB1C3) did not make any colony.<br> | ||
| - | + | LacP-Kil-DT 1,2,3,4,5,6,7,8 was failed.<br><br> | |
====Liquid culture==== | ====Liquid culture==== | ||
| - | + | LacP-torA-GFP-DT<br> | |
==July 23== | ==July 23== | ||
| Line 1,722: | Line 1,741: | ||
====Restriction Enzyme Processing ==== | ====Restriction Enzyme Processing ==== | ||
{| class="wikitable" | {| class="wikitable" | ||
| - | ! | + | !torA-GFP+DT||10×M Buffer||BSA||MilliQ||Xba1||Pst1||Total |
|- | |- | ||
|2.5||3.0||0.5||23.4||0.3||0.3||30.0 | |2.5||3.0||0.5||23.4||0.3||0.3||30.0 | ||
| Line 1,732: | Line 1,751: | ||
====Restriction Enzyme Processing==== | ====Restriction Enzyme Processing==== | ||
{|class="wikitable" | {|class="wikitable" | ||
| - | ! | + | !LacP+kil+DT ||BSA||10×H Buffer||EcoR1||Pst1||MilliQ||Total |
|- | |- | ||
|5.0||0.5||3.0||0.5||0.5||20.5||30.0 | |5.0||0.5||3.0||0.5||0.5||20.5||30.0 | ||
|}37℃ 2h | |}37℃ 2h | ||
{|class="wikitable" | {|class="wikitable" | ||
| - | ! | + | !LacP middle copy ||BSA||10×H Buffer||EcoR1||Pst1||MilliQ||Total |
|- | |- | ||
|10.0||0.5||3.0||0.5||0.5||15.5||30.0 | |10.0||0.5||3.0||0.5||0.5||15.5||30.0 | ||
| Line 1,749: | Line 1,768: | ||
==August 2== | ==August 2== | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
==August 3== | ==August 3== | ||
====Restriction Enzyme processing==== | ====Restriction Enzyme processing==== | ||
| Line 1,775: | Line 1,784: | ||
====Ligation==== | ====Ligation==== | ||
{|class="wikitable" | {|class="wikitable" | ||
| - | ! | + | !LacP+kil+DT||pSB4K5||ligation high||Total |
|- | |- | ||
|15||15||15||45 | |15||15||15||45 | ||
| Line 1,787: | Line 1,796: | ||
==August 4== | ==August 4== | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
==August 5== | ==August 5== | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
==August 6== | ==August 6== | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
==August 7== | ==August 7== | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
==August 8== | ==August 8== | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
==August 9== | ==August 9== | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
==August 10== | ==August 10== | ||
====Ligation==== | ====Ligation==== | ||
| Line 1,861: | Line 1,810: | ||
====Transformation==== | ====Transformation==== | ||
*RBS+GFP+DT | *RBS+GFP+DT | ||
| - | * | + | *LacP+kil+DT |
*RBS(for control) | *RBS(for control) | ||
:To put 2ng DNA in 20μL competent cell and leave it on ice for 30min | :To put 2ng DNA in 20μL competent cell and leave it on ice for 30min | ||
| Line 1,869: | Line 1,818: | ||
==August 11== | ==August 11== | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
==August 12== | ==August 12== | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
==August 13== | ==August 13== | ||
====Liquid culture==== | ====Liquid culture==== | ||
| Line 1,901: | Line 1,830: | ||
:B0034 76.5μg/mL | :B0034 76.5μg/mL | ||
====Colony PCR==== | ====Colony PCR==== | ||
| - | + | LacP+kil+DT ×5 | |
{|class="wikitable" | {|class="wikitable" | ||
!2×Quick Taq||VF||VF||MilliQ||Total | !2×Quick Taq||VF||VF||MilliQ||Total | ||
| Line 1,918: | Line 1,847: | ||
=====positive control(GFP+DT)===== | =====positive control(GFP+DT)===== | ||
=====negative control(MilliQ)===== | =====negative control(MilliQ)===== | ||
| - | =====① ~⑥ colony PCR | + | =====① ~⑥ colony PCR LacP+kil+DT===== |
次の写真 | 次の写真 | ||
=====① 1kb ladder ===== | =====① 1kb ladder ===== | ||
=====② Positive control (GFP+DT)===== | =====② Positive control (GFP+DT)===== | ||
=====③ Negative control (MilliQ)===== | =====③ Negative control (MilliQ)===== | ||
| - | =====④ | + | =====④ ~⑨ LacP+kil+DT ①~⑥===== |
=====⑩~⑫ pspA 10μL ,6×buffer 2μL===== | =====⑩~⑫ pspA 10μL ,6×buffer 2μL===== | ||
=====⑬ 1kb ladder===== | =====⑬ 1kb ladder===== | ||
| Line 1,942: | Line 1,871: | ||
==August 16== | ==August 16== | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
==August 17== | ==August 17== | ||
====Colony PCR==== | ====Colony PCR==== | ||
| Line 1,968: | Line 1,887: | ||
68℃, 1min<br> | 68℃, 1min<br> | ||
→35cycles<br><br> | →35cycles<br><br> | ||
| + | |||
====Electrophoresis==== | ====Electrophoresis==== | ||
| - | + | [[File:0820.JPG|300px|thumb|right]] | |
| + | |||
=====①1kb ladder===== | =====①1kb ladder===== | ||
=====②, ③ MgSO4 3μL, pspA===== | =====②, ③ MgSO4 3μL, pspA===== | ||
| Line 1,975: | Line 1,896: | ||
=====⑥ negative control(MilliQ)===== | =====⑥ negative control(MilliQ)===== | ||
=====⑧ 1kb ladder===== | =====⑧ 1kb ladder===== | ||
| - | + | <br><br><br><br><br> | |
==August 18== | ==August 18== | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
==August 19== | ==August 19== | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
==August 20== | ==August 20== | ||
====Restriction==== | ====Restriction==== | ||
| Line 2,036: | Line 1,937: | ||
|} | |} | ||
====Electrophoresis==== | ====Electrophoresis==== | ||
| - | + | ||
| + | [[File:0821e.jpg|300px|thumb|right]] | ||
| + | |||
=====① 1kb ladder===== | =====① 1kb ladder===== | ||
=====②, ③ torA-GFP-DT(Xba1,Pst1)===== | =====②, ③ torA-GFP-DT(Xba1,Pst1)===== | ||
| - | =====④, ⑤ J23113(Spe1,Pst1)===== | + | =====④, ⑤ J23113(Spe1,Pst1)===== |
| + | |||
====Transformation==== | ====Transformation==== | ||
| - | * | + | *LacP-torA-GFP-DT(Backbone pSB3C5) |
*BBa_K11704(Backbone pSB1A2) | *BBa_K11704(Backbone pSB1A2) | ||
====Colony PCR==== | ====Colony PCR==== | ||
| + | |||
| + | [[File:0821r.jpg|300px|thumb|right]] | ||
*pspA | *pspA | ||
{|class="wikitable" | {|class="wikitable" | ||
| Line 2,066: | Line 1,972: | ||
98℃, 10sec<br> | 98℃, 10sec<br> | ||
60℃, 30sec<br> | 60℃, 30sec<br> | ||
| - | 68℃, 30sec<br> | + | 68℃, 30sec<br> |
| + | |||
====Transformation==== | ====Transformation==== | ||
| - | * | + | *LacP-torA-GFP-DT(Backbone pSB3C5) |
*J23107-tatABCD | *J23107-tatABCD | ||
====PCR==== | ====PCR==== | ||
| Line 2,094: | Line 2,001: | ||
27.2μg/mL | 27.2μg/mL | ||
====Transformation(the second time)==== | ====Transformation(the second time)==== | ||
| - | * | + | *LacP-torA-GFP-DT(Backbone pSB3C5) |
*J23107-tatABCD | *J23107-tatABCD | ||
====Electrophoresis==== | ====Electrophoresis==== | ||
| Line 2,107: | Line 2,014: | ||
7. negative control for 5&6<br> | 7. negative control for 5&6<br> | ||
====PCR==== | ====PCR==== | ||
| - | + | LacP-GFP-DT(a kit of biological parts) | |
{|class="wikitable" | {|class="wikitable" | ||
!10×Buffer||2mM dNTPs||25mM MgSO4||VF||VR||KOD Plus Neo||MilliQ||DNA||Total | !10×Buffer||2mM dNTPs||25mM MgSO4||VF||VR||KOD Plus Neo||MilliQ||DNA||Total | ||
| Line 2,183: | Line 2,090: | ||
→Gel extraction | →Gel extraction | ||
====Electrophoresis==== | ====Electrophoresis==== | ||
| - | + | [[File:0824g.jpg|200px|thumb|right]]<br> | |
1. 1kb ladder<br> | 1. 1kb ladder<br> | ||
2. J23107-tatABCD →See "August 23 -PCR-"<br> | 2. J23107-tatABCD →See "August 23 -PCR-"<br> | ||
3. negative control for J23107-tatABCD<br> | 3. negative control for J23107-tatABCD<br> | ||
| - | 4. pSB1C3(EcoR1, Pst1)<br> | + | 4. pSB1C3(EcoR1, Pst1)<br> |
| + | |||
====Ligation==== | ====Ligation==== | ||
{| class="wikitable" | {| class="wikitable" | ||
| Line 2,235: | Line 2,143: | ||
68℃, 30sec<br> | 68℃, 30sec<br> | ||
→30cycles<br> | →30cycles<br> | ||
| - | + | [[File:0827c.jpg|200px|thumb|right]] | |
====Purification of PCR products==== | ====Purification of PCR products==== | ||
| Line 2,241: | Line 2,149: | ||
====Restriction==== | ====Restriction==== | ||
{| class="wikitable" | {| class="wikitable" | ||
| - | ! | + | !LacP(Backbone:pSB3C5)||Spe1||Pst1||BufferH||BSA||MilliQ||total |
|- | |- | ||
|20||0.5||0.5||3||0.5||5.5||30 | |20||0.5||0.5||3||0.5||5.5||30 | ||
| Line 2,272: | Line 2,180: | ||
|- | |- | ||
|5||5||3||1.5||1.5||1||33||50 | |5||5||3||1.5||1.5||1||33||50 | ||
| - | |} | + | |}<br> |
| - | + | [[File:0828c.jpg|200px|thumb|right]] | |
| + | |||
====Transformation==== | ====Transformation==== | ||
*pspA-pSB1C3 | *pspA-pSB1C3 | ||
| Line 2,281: | Line 2,190: | ||
*Kil-6 | *Kil-6 | ||
*Kil-7 →Miniprep 77.7μg/mL | *Kil-7 →Miniprep 77.7μg/mL | ||
| - | * | + | *LacP-torA-GFP-1 →Miniprep 88.3μg/mL |
| - | * | + | *LacP-torA-GFP-2 →Miniprep 85.0μg/mL |
| - | * | + | *LacP-torA-GFP-3 →Miniprep +++ |
==August 29== | ==August 29== | ||
| Line 2,293: | Line 2,202: | ||
====Restriction==== | ====Restriction==== | ||
{| class="wikitable" | {| class="wikitable" | ||
| - | ! | + | !LacP-Kil-DT(134.7ng/μL)||EcoR1||Spe1||BufferM||MilliQ||total |
|- | |- | ||
|10||1||1||3||15||30 | |10||1||1||3||15||30 | ||
| Line 2,299: | Line 2,208: | ||
37℃, overnight | 37℃, overnight | ||
====Colony PCR==== | ====Colony PCR==== | ||
| - | * | + | *LacP-torA-GFP-DT (1-5) |
*pspA-pSB1C3 (1-5) | *pspA-pSB1C3 (1-5) | ||
*pspA-pSB3C5 (1-6) | *pspA-pSB3C5 (1-6) | ||
| Line 2,314: | Line 2,223: | ||
====Gel extraction==== | ====Gel extraction==== | ||
J23107-tatABCD(EcoR1, Pst1) -5.9μg/mL | J23107-tatABCD(EcoR1, Pst1) -5.9μg/mL | ||
| + | [[File:0829.jpg|none|200px|thumb|right]] | ||
==August 30== | ==August 30== | ||
| Line 2,326: | Line 2,236: | ||
J23107-tatABCD-DT (Amp resistance) | J23107-tatABCD-DT (Amp resistance) | ||
====Electrophoresis==== | ====Electrophoresis==== | ||
| - | + | ||
| - | 1. | + | [[File:0830e.jpg|none|200px|thumb|right]]<br> |
| - | 2. | + | 1. LacP-torA-GFP-DT-1<br> |
| - | 3. | + | 2. LacP-torA-GFP-DT-2<br> |
| - | 4. | + | 3. LacP-torA-GFP-DT-3<br> |
| - | 5. | + | 4. LacP-torA-GFP-DT-4<br> |
| + | 5. LacP-torA-GFP-DT-5<br> | ||
6. pspA-pSB1C3-1<br> | 6. pspA-pSB1C3-1<br> | ||
7. pspA-pSB1C3-2<br> | 7. pspA-pSB1C3-2<br> | ||
| Line 2,343: | Line 2,254: | ||
15.pspA-pSB3C5-5<br> | 15.pspA-pSB3C5-5<br> | ||
16.pspA-pSB3C5-6<br> | 16.pspA-pSB3C5-6<br> | ||
| + | |||
====Miniprep==== | ====Miniprep==== | ||
*pspA-pSB3C5-1 176μg/mL | *pspA-pSB3C5-1 176μg/mL | ||
| Line 2,349: | Line 2,261: | ||
*pspA-pSB1C3-2 79μg/mL | *pspA-pSB1C3-2 79μg/mL | ||
====Colony PCR==== | ====Colony PCR==== | ||
| - | + | LacP-torA-GFP-DT (6-13) | |
{|class="wikitable" | {|class="wikitable" | ||
!2×Quick Taq||VF||VR||MilliQ||Total | !2×Quick Taq||VF||VR||MilliQ||Total | ||
| Line 2,368: | Line 2,280: | ||
at 37℃ for 1h | at 37℃ for 1h | ||
====Electrophoresis==== | ====Electrophoresis==== | ||
| - | + | [[File:0830r.jpg|none|200px|thumb|right]]<br> | |
1. 1kb ladder<br> | 1. 1kb ladder<br> | ||
3. pspA-pSB1C3(EcoR1, Spe1)<br> | 3. pspA-pSB1C3(EcoR1, Spe1)<br> | ||
| Line 2,374: | Line 2,286: | ||
==August 31== | ==August 31== | ||
====Gel extraction==== | ====Gel extraction==== | ||
| - | + | [[File:0831e.jpg|none|200px|thumb|right]]<br> | |
pspA-pSB1C3(EcoR1, Spe1) 8.0μg/mL | pspA-pSB1C3(EcoR1, Spe1) 8.0μg/mL | ||
| + | |||
====Purification==== | ====Purification==== | ||
| - | + | LacP-GFP-DT -0.7μg/mL | |
====Ligation==== | ====Ligation==== | ||
{| class="wikitable" | {| class="wikitable" | ||
| Line 2,417: | Line 2,330: | ||
at 4℃ for overnight | at 4℃ for overnight | ||
====Electrophoresis==== | ====Electrophoresis==== | ||
| - | + | [[File:0902.jpg|none|200px|thumb|right]]<br> | |
1. 1kb ladder<br> | 1. 1kb ladder<br> | ||
3. J23107-tatABCD-DT-1<br> | 3. J23107-tatABCD-DT-1<br> | ||
| Line 2,426: | Line 2,339: | ||
8. J23107-tatABCD-DT-7<br> | 8. J23107-tatABCD-DT-7<br> | ||
9. J23107-tatABCD-DT-8<br> | 9. J23107-tatABCD-DT-8<br> | ||
| - | + | [[File:0902-2.jpg|none|200px|thumb|right]]<br> | |
1. 1kb ladder<br> | 1. 1kb ladder<br> | ||
3. J23107-tatABCD-DT-1<br> | 3. J23107-tatABCD-DT-1<br> | ||
| Line 2,436: | Line 2,349: | ||
9. J23107-tatABCD-DT-8<br> | 9. J23107-tatABCD-DT-8<br> | ||
11. LacP-pSB3C5(Spe1, Pst1) | 11. LacP-pSB3C5(Spe1, Pst1) | ||
| - | + | [[File:0902-3.jpg|none|200px|thumb|right]]<br> | |
1. 1kb ladder<br> | 1. 1kb ladder<br> | ||
3. pspA-DT-2<br> | 3. pspA-DT-2<br> | ||
| Line 2,455: | Line 2,368: | ||
|} | |} | ||
====Gel extraction==== | ====Gel extraction==== | ||
| - | + | [[File:0903.jpg|none|200px|thumb|right]]<br> | |
LacP-torA-GFP-DT →See "September 5 -Gel extraction-" | LacP-torA-GFP-DT →See "September 5 -Gel extraction-" | ||
| + | |||
====Restriction==== | ====Restriction==== | ||
{| class="wikitable" | {| class="wikitable" | ||
| Line 2,466: | Line 2,380: | ||
J23107-tatABCD 29.6μg/mL 1.56(260/280) 1.60(260/230) | J23107-tatABCD 29.6μg/mL 1.56(260/280) 1.60(260/230) | ||
====Electrophoresis==== | ====Electrophoresis==== | ||
| - | + | [[File:0903-2.jpg|none|200px|thumb|right]]<br> | |
1. 1kb ladder<br> | 1. 1kb ladder<br> | ||
2. J23107-tatABCD<br> | 2. J23107-tatABCD<br> | ||
| + | |||
====Liquid culture==== | ====Liquid culture==== | ||
*LacP-kil-DT(1) | *LacP-kil-DT(1) | ||
| Line 2,485: | Line 2,400: | ||
→30cycles<br><br> | →30cycles<br><br> | ||
====Gel extraction==== | ====Gel extraction==== | ||
| - | + | [[File:IMG_2135.jpg|none|200px|thumb|right]]<br> | |
LacP-torA-GFP-DT 31μg/mL 1.28(260/280) 0.49(260/230) | LacP-torA-GFP-DT 31μg/mL 1.28(260/280) 0.49(260/230) | ||
LacP-torA-GFP-DT 8μg/mL 1.32(260/280) 0.83(260/230) | LacP-torA-GFP-DT 8μg/mL 1.32(260/280) 0.83(260/230) | ||
| + | |||
====Transformation==== | ====Transformation==== | ||
J23107-tatABCD (Amp resistance)<br> | J23107-tatABCD (Amp resistance)<br> | ||
| Line 2,497: | Line 2,413: | ||
J23105-kil-DT (CP resistance)→We didn't transform because of lack of culture medium.<br> | J23105-kil-DT (CP resistance)→We didn't transform because of lack of culture medium.<br> | ||
====Electrophoresis==== | ====Electrophoresis==== | ||
| - | + | [[File:IMG_2136.jpg|none|200px|thumb|right]]<br> | |
1. 1kb ladder<br> | 1. 1kb ladder<br> | ||
2-7. LacP-torA-GFP-DT<br> | 2-7. LacP-torA-GFP-DT<br> | ||
| + | |||
====Colony PCR==== | ====Colony PCR==== | ||
LacP-torA-GFP-DT (14-21) | LacP-torA-GFP-DT (14-21) | ||
| Line 2,527: | Line 2,444: | ||
→30cycles<br><br> | →30cycles<br><br> | ||
====Electrophoresis==== | ====Electrophoresis==== | ||
| - | + | [[File:IMG_2137.jpg|none|200px|thumb|right]]<br> | |
| - | 1-8. | + | 1-8. LacP-torA-GFP-DT<br> |
| - | + | [[File:IMG_2138.jpg|none|200px|thumb|right]]<br> | |
1-4. J23113-kil-DT (Amp resistance)<br> | 1-4. J23113-kil-DT (Amp resistance)<br> | ||
5-8. J23113-kil-DT (CP resistance)<br> | 5-8. J23113-kil-DT (CP resistance)<br> | ||
| + | |||
====Liquid culture==== | ====Liquid culture==== | ||
*LacP-torA-GFP-DT | *LacP-torA-GFP-DT | ||
| Line 2,560: | Line 2,478: | ||
==September 5== | ==September 5== | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
====Colony PCR==== | ====Colony PCR==== | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
J23107-tatABCD (1-7) | J23107-tatABCD (1-7) | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
{|class="wikitable" | {|class="wikitable" | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
!buffer||2mM dNTPs||25mM MgSO4||primer-f||primer-r||KOD Plus Neo||DW||Total | !buffer||2mM dNTPs||25mM MgSO4||primer-f||primer-r||KOD Plus Neo||DW||Total | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
|- | |- | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
|5||5||3||1.5||1.5||1||33||50 | |5||5||3||1.5||1.5||1||33||50 | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
|} | |} | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
94℃, 2min<br> | 94℃, 2min<br> | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
98℃, 10sec<br> | 98℃, 10sec<br> | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
60℃, 30sec<br> | 60℃, 30sec<br> | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
68℃, 1.25min<br> | 68℃, 1.25min<br> | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
→30cycles<br><br> | →30cycles<br><br> | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
====Gel extraction==== | ====Gel extraction==== | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
<small>by Kato</small><br> | <small>by Kato</small><br> | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
See "September 3 -Gel extraction-" | See "September 3 -Gel extraction-" | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
LacP-torA-GFP-DT 15.9μg/mL 1.29(260/280) 0.38(260/230) | LacP-torA-GFP-DT 15.9μg/mL 1.29(260/280) 0.38(260/230) | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
LacP-torA-GFP-DT 17.2μg/mL 1.23(260/280) 0.48(260/230) | LacP-torA-GFP-DT 17.2μg/mL 1.23(260/280) 0.48(260/230) | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
====Miniprep==== | ====Miniprep==== | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
*J23113-kil-DT 85.7μg/mL 1.76(260/280) 1.97(260/230) | *J23113-kil-DT 85.7μg/mL 1.76(260/280) 1.97(260/230) | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
*LacP-torA-GFP-DT 126.6μg/mL 1.81(260/280) 2.09(260/230) | *LacP-torA-GFP-DT 126.6μg/mL 1.81(260/280) 2.09(260/230) | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
*LacP-kil-DT-4 62.5μg/mL 1.79(260/280) 1.99(260/230) | *LacP-kil-DT-4 62.5μg/mL 1.79(260/280) 1.99(260/230) | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
*LacP-kil-DT-5 56.0μg/mL 1.79(260/280) 2.01(260/230) | *LacP-kil-DT-5 56.0μg/mL 1.79(260/280) 2.01(260/230) | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
*pspA-DT-3 322μg/mL 1.45(260/280) 1.42(260/230) | *pspA-DT-3 322μg/mL 1.45(260/280) 1.42(260/230) | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
*pspA-DT-6 147.6μg/mL 1.25(260/280) 1.37(260/230) | *pspA-DT-6 147.6μg/mL 1.25(260/280) 1.37(260/230) | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
*pspA-DT-7 145.5μg/mL 1.24(260/280) 1.27(260/230) | *pspA-DT-7 145.5μg/mL 1.24(260/280) 1.27(260/230) | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
====Restriction==== | ====Restriction==== | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
{| class="wikitable" | {| class="wikitable" | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
!pspA-DT-6||Xba1||Pst1||BufferM||BSA||MilliQ||total | !pspA-DT-6||Xba1||Pst1||BufferM||BSA||MilliQ||total | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
|- | |- | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
|20||0.5||0.5||3||0.5||5.5||30 | |20||0.5||0.5||3||0.5||5.5||30 | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
|} | |} | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
at 37℃ for 1h | at 37℃ for 1h | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
====Electrophoresis==== | ====Electrophoresis==== | ||
| - | + | [[File:IMG_2139.jpg|none|200px|thumb|right]]<br> | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
J23107-tatABCD (1-7)<br> | J23107-tatABCD (1-7)<br> | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
====Colony PCR==== | ====Colony PCR==== | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
J23107-tatABCD (8-23) | J23107-tatABCD (8-23) | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
{|class="wikitable" | {|class="wikitable" | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
!2×Quick Taq||VF2||VR||MilliQ||Total | !2×Quick Taq||VF2||VR||MilliQ||Total | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
|- | |- | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
|25||1||1||23||50 | |25||1||1||23||50 | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
|} | |} | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
94℃, 2min<br> | 94℃, 2min<br> | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
94℃, 30sec<br> | 94℃, 30sec<br> | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
55℃, 30sec<br> | 55℃, 30sec<br> | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
68℃, 1.5min<br> | 68℃, 1.5min<br> | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
→30cycles<br><br> | →30cycles<br><br> | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
====Gel extraction==== | ====Gel extraction==== | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
pspA-DT 10.2μg/mL 1.42(260/280) 0.76(260/230) | pspA-DT 10.2μg/mL 1.42(260/280) 0.76(260/230) | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
====Ligation==== | ====Ligation==== | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
{| class="wikitable" | {| class="wikitable" | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
!pspA-DT(Xba1, Pst1)||LacP(pSB3C5)(Spe1, Pst1)||Ligation High Ver.2||total | !pspA-DT(Xba1, Pst1)||LacP(pSB3C5)(Spe1, Pst1)||Ligation High Ver.2||total | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
|- | |- | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
|5||1||3||9 | |5||1||3||9 | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
|} | |} | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
at 16℃ | at 16℃ | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
====Electrophoresis==== | ====Electrophoresis==== | ||
| - | + | [[File:IMG_2140.jpg|none|200px|thumb|right]]<br> | |
| - | + | LacP-torA-GFP-DT(8-15)<br> | |
| - | + | [[File:IMG_2141.jpg|none|200px|thumb|right]]<br> | |
| - | + | LacP-torA-GFP-DT(9-23)<br> | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
====Ligation==== | ====Ligation==== | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
{| class="wikitable" | {| class="wikitable" | ||
| - | + | !LacP-torA-GFP-DT(EcoR1, Spe1)||DT 17.2μg/mL(Xba1, Pst1)||Ligation High Ver.2||total | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | ! | + | |
| - | + | ||
| - | + | ||
|- | |- | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
|15||25||20||60 | |15||25||20||60 | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
|} | |} | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
====Liquid culture==== | ====Liquid culture==== | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
*J23107-tatABCD-8 | *J23107-tatABCD-8 | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
*J23107-tatABCD-20 | *J23107-tatABCD-20 | ||
| - | + | *LacP-torA-GFP-DT-21 | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | * | + | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
==September 6== | ==September 6== | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
====Miniprep==== | ====Miniprep==== | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
*J23107-tatABCD-8 82.4μg/mL 2.06(260/280) 2.95(260/230) | *J23107-tatABCD-8 82.4μg/mL 2.06(260/280) 2.95(260/230) | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
*J23107-tatABCD-20 102.9μg/mL 2.04(260/280) 2.94(260/230) | *J23107-tatABCD-20 102.9μg/mL 2.04(260/280) 2.94(260/230) | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
====Restriction==== | ====Restriction==== | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
{| class="wikitable" | {| class="wikitable" | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
!J23107-tatABCD 102.9μg/mL||Spe1||Pst1||BufferH||BSA||MilliQ||total | !J23107-tatABCD 102.9μg/mL||Spe1||Pst1||BufferH||BSA||MilliQ||total | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
|- | |- | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
|10||0.5||0.5||3||0.5||15.5||30 | |10||0.5||0.5||3||0.5||15.5||30 | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
|} | |} | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
at 37℃ for 1h | at 37℃ for 1h | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
====Transformation==== | ====Transformation==== | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
LacP-pspA-DT(pSB3C5) | LacP-pspA-DT(pSB3C5) | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
{|class="wikitable" | {|class="wikitable" | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
!DNA||Competent cell||total | !DNA||Competent cell||total | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
|- | |- | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
|2||20||22 | |2||20||22 | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
|} | |} | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
====Gel extraction==== | ====Gel extraction==== | ||
| - | + | [[File:IMG_2142.jpg|none|200px|thumb|right]]<br> | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
J23107-tatABCD(Spe1, Pst1) | J23107-tatABCD(Spe1, Pst1) | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
→We failed gel extraction because we mistook steps of experiment. | →We failed gel extraction because we mistook steps of experiment. | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
====Electrophoresis==== | ====Electrophoresis==== | ||
| - | + | [[File:IMG_2143.jpg|none|200px|thumb|right]]<br> | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
LacP-torA-GFP-DT-pspA-DT<br> | LacP-torA-GFP-DT-pspA-DT<br> | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
====Restriction==== | ====Restriction==== | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
<small>by Terasaka</small><br> | <small>by Terasaka</small><br> | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
{| class="wikitable" | {| class="wikitable" | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
!J23107-tatABCD||Spe1||Pst1||BufferH||BSA||MilliQ||total | !J23107-tatABCD||Spe1||Pst1||BufferH||BSA||MilliQ||total | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
|- | |- | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
|10||0.5||0.5||3||0.5||15.5||30 | |10||0.5||0.5||3||0.5||15.5||30 | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
|} | |} | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
====Gel extraction==== | ====Gel extraction==== | ||
| - | + | [[File:IMG_2144.jpg|none|200px|thumb|right]]<br> | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
J23107-tatABCD(Spe1, Pst1) | J23107-tatABCD(Spe1, Pst1) | ||
| - | + | →See "September 7 -Gel extraction-" | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | →See "September 7 -Gel extraction-" | + | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
==September 7== | ==September 7== | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
====Liquid culture==== | ====Liquid culture==== | ||
| - | + | *LacP-torA-GFP-DT-1 (CP resistance) | |
| - | + | *LacP-torA-GFP-DT-21 (CP resistance) | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | * | + | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | * | + | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
preculture for 1 hour | preculture for 1 hour | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
====Colony PCR==== | ====Colony PCR==== | ||
| - | + | LacP-torA-GFP-DT (1-8) | |
| - | + | LacP-pspA-DT (1-8) | |
{|class="wikitable" | {|class="wikitable" | ||
!2×Quick Taq||VF2||VR||MilliQ||Total | !2×Quick Taq||VF2||VR||MilliQ||Total | ||
| Line 3,834: | Line 2,607: | ||
68℃, 1.5min<br> | 68℃, 1.5min<br> | ||
→30cycles<br><br> | →30cycles<br><br> | ||
| - | |||
====Restriction==== | ====Restriction==== | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
<small>by Terasaka</small><br> | <small>by Terasaka</small><br> | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
{| class="wikitable" | {| class="wikitable" | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
!J23107-tatABCD||EcoR1||Spe1||BufferM||BSA||MilliQ||total | !J23107-tatABCD||EcoR1||Spe1||BufferM||BSA||MilliQ||total | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
|- | |- | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
|10||0.5||0.5||3||0.5||15.5||30 | |10||0.5||0.5||3||0.5||15.5||30 | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
|} | |} | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
{| class="wikitable" | {| class="wikitable" | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
!GFP-DT||Xba1||Pst1||BufferM||BSA||MilliQ||total | !GFP-DT||Xba1||Pst1||BufferM||BSA||MilliQ||total | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
|- | |- | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
|15||0.5||0.5||3||0.5||10.5||30 | |15||0.5||0.5||3||0.5||10.5||30 | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
|} | |} | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
====Electrophoresis==== | ====Electrophoresis==== | ||
| - | + | [[File:IMG_2145.jpg|none|200px|thumb|right]]<br> | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
① -⑧. LacP-pspA-DT<br> | ① -⑧. LacP-pspA-DT<br> | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
1-8. LacP-torA-GFP-DT<br> | 1-8. LacP-torA-GFP-DT<br> | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
====Gel extraction==== | ====Gel extraction==== | ||
| - | + | [[File:IMG_2146.jpg|none|200px|thumb|right]] | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
*J23107-tatABCD(EcoR1, Spe1) | *J23107-tatABCD(EcoR1, Spe1) | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
*J23107-tatABCD(Pst1, Spe1) ①57.6μg/mL ②23.4μg/mL | *J23107-tatABCD(Pst1, Spe1) ①57.6μg/mL ②23.4μg/mL | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
*GFP-DT(Xba1, Pst1) | *GFP-DT(Xba1, Pst1) | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
====Restriction==== | ====Restriction==== | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
{| class="wikitable" | {| class="wikitable" | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
!pspA-DT(145.5μg/mL)||Xba1||Pst1||BufferM||BSA||MilliQ||total | !pspA-DT(145.5μg/mL)||Xba1||Pst1||BufferM||BSA||MilliQ||total | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
|- | |- | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
|20||0.5||0.5||3||0.5||5.5||30 | |20||0.5||0.5||3||0.5||5.5||30 | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
|} | |} | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
37℃, overnight | 37℃, overnight | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
====Liquid culture==== | ====Liquid culture==== | ||
| - | + | *LacP-torA-GFP-DT-7 (LB 2mL)<br> | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | * | + | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
+IPTG 0mM, 0.05mM, 0.1mM, 0.15mM, 0.2mM, 0.3mM | +IPTG 0mM, 0.05mM, 0.1mM, 0.15mM, 0.2mM, 0.3mM | ||
| - | + | *LacP-torA-GFP-DT-7 (LB 5mL) | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | * | + | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
==September 8== | ==September 8== | ||
====Check the effect of Amp==== | ====Check the effect of Amp==== | ||
| - | We used 5mL of the liquid culture medium with | + | We used 5mL of the liquid culture medium with LacP-torA-GFP-DT-7.<br> |
→See "September 7 -Liquid culture-"<br> | →See "September 7 -Liquid culture-"<br> | ||
We diluted it with LB until OD600=0.444, and dispensed it.<br> | We diluted it with LB until OD600=0.444, and dispensed it.<br> | ||
| Line 4,201: | Line 2,654: | ||
|} | |} | ||
====Observation through a confocal microscope==== | ====Observation through a confocal microscope==== | ||
| - | We used the liquid culture media with | + | We used the liquid culture media with LacP-torA-GFP-DT + 13μL of Amp(50mg/mL) + IPTG 0/0.1/0.15mM.<br> |
Their OD600 was 0.47.<br> | Their OD600 was 0.47.<br> | ||
3h after incubating at 37℃, we eliminated each supernatant using a centrifuge, and diluted them with LB to which Amp was added.<br> | 3h after incubating at 37℃, we eliminated each supernatant using a centrifuge, and diluted them with LB to which Amp was added.<br> | ||
| Line 4,208: | Line 2,661: | ||
====Gel extraction==== | ====Gel extraction==== | ||
pspA-DT -5.5μg/mL | pspA-DT -5.5μg/mL | ||
| + | [[File:IMG_2147.jpg|none|200px|thumb|right]] | ||
====Restriction==== | ====Restriction==== | ||
{| class="wikitable" | {| class="wikitable" | ||
| Line 4,224: | Line 2,678: | ||
|} | |} | ||
====Electrophoresis==== | ====Electrophoresis==== | ||
| - | + | [[File:IMG_2148.jpg|none|200px|thumb|right]]<br> | |
1. J23107-tatABCD(EcoR1, Spe1)<br> | 1. J23107-tatABCD(EcoR1, Spe1)<br> | ||
2. J23107-tatABCD(before restriction)<br> | 2. J23107-tatABCD(before restriction)<br> | ||
| Line 4,230: | Line 2,684: | ||
4. pspA-DT(before restriction)<br> | 4. pspA-DT(before restriction)<br> | ||
====Gel extraction==== | ====Gel extraction==== | ||
| - | + | [[File:IMG_2149.jpg|none|200px|thumb|right]]<br> | |
*J23107-tatABCD(EcoR1, Spe1) 26μg/mL | *J23107-tatABCD(EcoR1, Spe1) 26μg/mL | ||
*pspA-DT(Xba1, Pst1) 199μg/mL | *pspA-DT(Xba1, Pst1) 199μg/mL | ||
| Line 4,236: | Line 2,690: | ||
*J23100/J23101/J23106-1 | *J23100/J23101/J23106-1 | ||
*pspA-DT-5 ×2 | *pspA-DT-5 ×2 | ||
| - | * | + | *LacP-torA-GFP-DT-21 |
*GFP generator-2 | *GFP generator-2 | ||
| - | * | + | *LacP-pspA-DT-6 |
==September 10== | ==September 10== | ||
| Line 4,246: | Line 2,700: | ||
*J23106-1 90.9μg/mL | *J23106-1 90.9μg/mL | ||
*pspA-DT-5 ①104.1μg/mL ②80.0μg/mL | *pspA-DT-5 ①104.1μg/mL ②80.0μg/mL | ||
| - | * | + | *LacP-torA-GFP-DT-21 79.6μg/mL |
*GFP generator-2 84.1μg/mL | *GFP generator-2 84.1μg/mL | ||
| - | * | + | *LacP-pspA-DT-6 99.8μg/mL |
====Ligation==== | ====Ligation==== | ||
*J23107-tatABCD-DT | *J23107-tatABCD-DT | ||
| Line 4,275: | Line 2,729: | ||
|} | |} | ||
====Restriction==== | ====Restriction==== | ||
| - | + | [[File:IMG_2151.jpg|none|200px|thumb|right]] | |
{| class="wikitable" | {| class="wikitable" | ||
!DNA||Spe1||Pst1||BufferH||MilliQ||total | !DNA||Spe1||Pst1||BufferH||MilliQ||total | ||
| Line 4,284: | Line 2,738: | ||
at 37℃ | at 37℃ | ||
====Gel extraction==== | ====Gel extraction==== | ||
| - | + | [[File:IMG_2152.jpg|none|200px|thumb|right]] | |
| + | [[File:IMG_2153.jpg|none|200px|thumb|right]]<br> | ||
J23100 27.1μg/mL<br> | J23100 27.1μg/mL<br> | ||
J23101 50.2μg/mL<br> | J23101 50.2μg/mL<br> | ||
| Line 4,319: | Line 2,774: | ||
→30cycles | →30cycles | ||
====Electrophoresis==== | ====Electrophoresis==== | ||
| - | + | [[File:IMG_2155.jpg|none|200px|thumb|right]] | |
| + | [[File:IMG_2156.jpg|none|200px|thumb|right]] | ||
*J23107-tatABCD-DT (1-8) | *J23107-tatABCD-DT (1-8) | ||
*J23107-tatABCD-pspA-DT (1-8) | *J23107-tatABCD-pspA-DT (1-8) | ||
| + | |||
====Restriction==== | ====Restriction==== | ||
| - | + | [[File:IMG_2154.jpg|none|200px|thumb|right]] | |
{| class="wikitable" | {| class="wikitable" | ||
!GFP generator(84.1μg/mL)||Xba1||Pst1||BufferM||BSA||MilliQ||total | !GFP generator(84.1μg/mL)||Xba1||Pst1||BufferM||BSA||MilliQ||total | ||
| Line 4,330: | Line 2,787: | ||
|} | |} | ||
{| class="wikitable" | {| class="wikitable" | ||
| - | ! | + | !LacP-torA-GFP-DT(85.0μg/mL)||Spe1||Pst1||BufferH||MilliQ||total |
|- | |- | ||
|15||1||1||3||10||30 | |15||1||1||3||10||30 | ||
|} | |} | ||
{| class="wikitable" | {| class="wikitable" | ||
| - | ! | + | !LacP-pspA-DT(99.8μg/mL)||Xba1||Pst1||BufferM||BSA||MilliQ||total |
|- | |- | ||
|15||1||1||3||3||7||30 | |15||1||1||3||3||7||30 | ||
|} | |} | ||
| + | |||
====Liquid culture==== | ====Liquid culture==== | ||
pSB1C3(self-ligation) ×5 in LB 3mLCP 3μL<br> | pSB1C3(self-ligation) ×5 in LB 3mLCP 3μL<br> | ||
| Line 4,373: | Line 2,831: | ||
|} | |} | ||
====Gel extraction==== | ====Gel extraction==== | ||
| - | + | [[File:IMG_2159.jpg|none|200px|thumb|right]] | |
*GFP generator (Xba1, Pst1) 14.1μg/mL | *GFP generator (Xba1, Pst1) 14.1μg/mL | ||
| - | * | + | *LacP-torA-GFP-DT (Spe1, Pst1) 42.9μg/mL 1.13(260/280) 1.19(260/230) |
| - | * | + | *LacP-pspA-DT (Xba1, Pst1) 20.0μg/mL 1.01(260/280) 1.30(260/230) |
| + | |||
====Electrophoresis==== | ====Electrophoresis==== | ||
| - | + | [[File:IMG_2160.jpg|none|200px|thumb|right]] | |
*J23107-tatABCD-DT (1-8) | *J23107-tatABCD-DT (1-8) | ||
*J23106-kil-DT (1, 2) | *J23106-kil-DT (1, 2) | ||
*J23100-kil-DT (1-4) | *J23100-kil-DT (1-4) | ||
| + | |||
====Liquid culture==== | ====Liquid culture==== | ||
| - | * | + | *LacP-torA-GFP-DT |
*pSB1C3 | *pSB1C3 | ||
====Colony PCR==== | ====Colony PCR==== | ||
We did PCR 10 more cycle to amplify products of PCR on 9/11 of J23107-tatABCD-pspA-DT and J23107-tatABCD-DT. | We did PCR 10 more cycle to amplify products of PCR on 9/11 of J23107-tatABCD-pspA-DT and J23107-tatABCD-DT. | ||
| + | |||
| + | |||
| + | |||
| + | |||
==September 12== | ==September 12== | ||
| Line 4,413: | Line 2,877: | ||
|} | |} | ||
====Electrophoresis==== | ====Electrophoresis==== | ||
| - | + | [[File:IMG_2162.jpg|none|200px|thumb|right]] | |
*J23107-tatABCD-pspA-DT | *J23107-tatABCD-pspA-DT | ||
| - | + | [[File:IMG_2161.jpg|none|200px|thumb|right]] | |
*J23101-kil-DT (1-7) | *J23101-kil-DT (1-7) | ||
| + | |||
====Restriction==== | ====Restriction==== | ||
{| class="wikitable" | {| class="wikitable" | ||
| - | !pspA( | + | !pspA(pSB3C5) (106μg/mL)||EcoR1||Pst1||BufferH||MilliQ||total |
|- | |- | ||
|15||1||1||3||10||30 | |15||1||1||3||10||30 | ||
| Line 4,434: | Line 2,899: | ||
|} | |} | ||
====Electrophoresis==== | ====Electrophoresis==== | ||
| - | + | [[File:IMG_2165.jpg|none|200px|thumb|right]] | |
| - | *pspA( | + | *pspA(pSB3C5)(EcoR1, Pst1) |
*pspA-DT(Xba1, Pst1) | *pspA-DT(Xba1, Pst1) | ||
*J23107-tatABCD(EcoR1, Spe1) | *J23107-tatABCD(EcoR1, Spe1) | ||
*pSB1C3(EcoR1, Spe1) | *pSB1C3(EcoR1, Spe1) | ||
| + | |||
====Transformation==== | ====Transformation==== | ||
J23107-tatABCD-pspA-DT(pSB3C5) | J23107-tatABCD-pspA-DT(pSB3C5) | ||
| Line 4,453: | Line 2,919: | ||
incubate CP culture plate | incubate CP culture plate | ||
====Gel extraction==== | ====Gel extraction==== | ||
| - | + | [[File:IMG_2166.jpg|none|200px|thumb|right]] | |
| - | *pspA( | + | *pspA(pSB3C5)(EcoR1, Pst1) 26.8μg/mL 1.27(260/280) 0.32(260/230) |
*pspA-DT(Xba1, Pst1) 26.1μg/mL 1.13(260/280) 0.55(260/230) | *pspA-DT(Xba1, Pst1) 26.1μg/mL 1.13(260/280) 0.55(260/230) | ||
*J23107-tatABCD(EcoR1, Spe1) 1.5μg/mL 1.99(260/280) 0.06(260/230) | *J23107-tatABCD(EcoR1, Spe1) 1.5μg/mL 1.99(260/280) 0.06(260/230) | ||
| + | |||
====Ligation==== | ====Ligation==== | ||
{| class="wikitable" | {| class="wikitable" | ||
| Line 4,527: | Line 2,994: | ||
==September 14== | ==September 14== | ||
====Colony PCR==== | ====Colony PCR==== | ||
| - | + | [[File:IMG_2169.jpg|none|200px|thumb|right]]<br> | |
See "September 13 -Colony PCR-"<br> | See "September 13 -Colony PCR-"<br> | ||
J23107-tatABCD-pspA-DT<br> | J23107-tatABCD-pspA-DT<br> | ||
| + | |||
====Colony PCR==== | ====Colony PCR==== | ||
*J23107-tatABCD-pspA-DT (1-6) | *J23107-tatABCD-pspA-DT (1-6) | ||
| Line 4,575: | Line 3,043: | ||
37℃, 2 hours | 37℃, 2 hours | ||
====Electrophoresis==== | ====Electrophoresis==== | ||
| - | + | [[File:IMG_2170.jpg|none|200px|thumb|right]]<br> | |
①. 1kb ladder<br> | ①. 1kb ladder<br> | ||
②. J23107-tatABCD-pspA-DT<br> | ②. J23107-tatABCD-pspA-DT<br> | ||
| Line 4,584: | Line 3,052: | ||
⑦. LacP-torA-GFP-DT<br> | ⑦. LacP-torA-GFP-DT<br> | ||
⑧. LacP-torA-GFP-DT(EcoR1, Xba1)<br> | ⑧. LacP-torA-GFP-DT(EcoR1, Xba1)<br> | ||
| + | |||
====Gel extraction==== | ====Gel extraction==== | ||
| - | + | [[File:IMG_2171.jpg|none|200px|thumb|right]] | |
| + | [[File:IMG_2172.jpg|none|200px|thumb|right]]<br> | ||
①. 1kb ladder<br> | ①. 1kb ladder<br> | ||
②. J23107-tatABCD-pspA-DT(EcoR1, Spe1)<br> | ②. J23107-tatABCD-pspA-DT(EcoR1, Spe1)<br> | ||
| Line 4,594: | Line 3,064: | ||
*J23107-tatABCD-pspA-DT(EcoR1, Spe1) 14.2μg/mL 1.28(260/280) 0.54(260/230) | *J23107-tatABCD-pspA-DT(EcoR1, Spe1) 14.2μg/mL 1.28(260/280) 0.54(260/230) | ||
*J23107-tatABCD-pspA-DT(EcoR1, Pst1) 7.3μg/mL 1.55(260/280) 0.38(260/230) | *J23107-tatABCD-pspA-DT(EcoR1, Pst1) 7.3μg/mL 1.55(260/280) 0.38(260/230) | ||
| + | |||
====Purification==== | ====Purification==== | ||
*LacP-torA-GFP-DT(EcoR1, Xba1) 7.7μg/mL 1.41(260/280) 0.99(260/230) | *LacP-torA-GFP-DT(EcoR1, Xba1) 7.7μg/mL 1.41(260/280) 0.99(260/230) | ||
| Line 4,631: | Line 3,102: | ||
|} | |} | ||
====Electrophoresis==== | ====Electrophoresis==== | ||
| - | + | [[File:IMG_2173.jpg|none|200px|thumb|right]]<br> | |
A1-A6. J23107-tatABCD-pspA-DT-LacP-torA-GFP-DT (1-6)<br> | A1-A6. J23107-tatABCD-pspA-DT-LacP-torA-GFP-DT (1-6)<br> | ||
B1-B6. J23107-tatABCD-pspA-DT(pSB1C3) (1-6)<br> | B1-B6. J23107-tatABCD-pspA-DT(pSB1C3) (1-6)<br> | ||
| + | |||
====Liquid culture==== | ====Liquid culture==== | ||
*J23107-tatABCD-pspA-DT(pSB1C3) | *J23107-tatABCD-pspA-DT(pSB1C3) | ||
| Line 4,648: | Line 3,120: | ||
lane7. LacP-torA-GFP-DT(EcoR1, Xba1)<br> | lane7. LacP-torA-GFP-DT(EcoR1, Xba1)<br> | ||
lane8. LacP-torA-GFP-DT(Xba1)<br> | lane8. LacP-torA-GFP-DT(Xba1)<br> | ||
| + | |||
====Miniprep==== | ====Miniprep==== | ||
*J23106-kil-DT 11.7μg/mL 1.93(260/280) 1.46(260/230) | *J23106-kil-DT 11.7μg/mL 1.93(260/280) 1.46(260/230) | ||
| Line 4,653: | Line 3,126: | ||
==September 18== | ==September 18== | ||
====Gel extraction==== | ====Gel extraction==== | ||
| - | + | [[File:IMG_2174.jpg|none|200px|thumb|right]] | |
*LacP-torA-GFP-DT(EcoR1, Xba1) 36.9μg/mL 1.37(260/280) 0.82(260/230) | *LacP-torA-GFP-DT(EcoR1, Xba1) 36.9μg/mL 1.37(260/280) 0.82(260/230) | ||
====Ligation==== | ====Ligation==== | ||
| Line 4,671: | Line 3,144: | ||
*LacP-torA-GFP-DT(EcoR1, Xba1) | *LacP-torA-GFP-DT(EcoR1, Xba1) | ||
====Observation through a confocal microscope==== | ====Observation through a confocal microscope==== | ||
| - | We made sample for observation through a confocal microscope | + | We made sample for observation through a confocal microscope.<br> |
| - | We incubated sample with 0mM/0.5mM IPTG for 1.5 hours. | + | We incubated sample with 0mM/0.5mM IPTG for 1.5 hours.<br> |
| - | Then, we incubated onto medium without IPTG for 2.5 hours. | + | Then, we incubated onto medium without IPTG for 2.5 hours.<br> |
{| class="wikitable" | {| class="wikitable" | ||
| Line 4,682: | Line 3,155: | ||
|0.1||LB | |0.1||LB | ||
|- | |- | ||
| - | |0.1||LB with 0.1mM IPTG | + | |0.1||LB with 0.1mM IPTG (positive control) |
|- | |- | ||
|0||LB with 2 percent glucose | |0||LB with 2 percent glucose | ||
| Line 4,692: | Line 3,165: | ||
|0.1||LB with 5 percent glucose | |0.1||LB with 5 percent glucose | ||
|- | |- | ||
| - | |0||LB with 2 percent glucose | + | |0||LB with 2 percent glucose (make medium new after incubate for 1.5 hours) |
|- | |- | ||
| - | |0||LB | + | |0||LB with 2 percent glucose (make medium new after incubate for 1.5 hours) |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
|} | |} | ||
| + | ====Restriction==== | ||
| + | {| class="wikitable" | ||
| + | !pSB4K5||EcoR1||Pst1||BufferH||MilliQ||total | ||
| + | |- | ||
| + | |10||1||1||3||15||30 | ||
| + | |} | ||
| + | {| class="wikitable" | ||
| + | !pSB4K5||EcoR1||BufferH||MilliQ||total | ||
| + | |- | ||
| + | |3||0.5||1||5.5||30 | ||
| + | |} | ||
| + | {| class="wikitable" | ||
| + | !pSB4K5||Pst1||BufferH||MilliQ||total | ||
| + | |- | ||
| + | |3||0.5||1||5.5||10 | ||
| + | |} | ||
| + | {| class="wikitable" | ||
| + | !LacP-torA-GFP-DT||Xba1||Pst1||BufferM||BSA||MilliQ||total | ||
| + | |- | ||
| + | |10||1||1||3||3||12||30 | ||
| + | |} | ||
| + | {| class="wikitable" | ||
| + | !LacP-torA-GFP-DT||Xba1||BufferM||BSA||MilliQ||total | ||
| + | |- | ||
| + | |2||1||1||1||5||10 | ||
| + | |} | ||
| + | {| class="wikitable" | ||
| + | !LacP-torA-GFP-DT||Pst1||BufferH||MilliQ||total | ||
| + | |- | ||
| + | |2||1||1||6||10 | ||
| + | |} | ||
| + | 37℃, 2 hours | ||
| + | ====Gel extraction==== | ||
| + | *LacP-torA-GFP-DT(Xba1, Pst1) 9.7μg/mL 1.34(260/280) 0.62(260/230) | ||
| + | *pSB4K5(EcoR1, Pst1) 9.6μg/mL 1.28(260/280) 0.32(260/230) | ||
| + | ====Miniprep==== | ||
| + | *J23107-tatABCD-psp-DT(pSB1C3) 78μg/mL | ||
| + | *J23107-tatABCD-psp-DT(pSB1C3) 48μg/mL | ||
| + | *LacP-torA-GFP-DT 29μg/mL | ||
| + | *J23101-kil-DT 25μg/mL | ||
| + | ====Restriction enzyme processing==== | ||
| + | |||
| + | {| class="wikitable" | ||
| + | !J23101-Kil-DT 25µg/ml||BufferM||Xba1||Pst1||BSA||MilliQ||total | ||
| + | |- | ||
| + | |40||6||1||1||6||6||60 | ||
| + | |} | ||
| + | {| class="wikitable" | ||
| + | !J23101-Kil-DT 25µg/ml||BufferM||Xba1||BSA||MilliQ||total | ||
| + | |- | ||
| + | |3||1||0.5||1||4.5||10 | ||
| + | |} | ||
| + | {| class="wikitable" | ||
| + | !J23101-Kil-DT 25µg/ml||BufferH||Pst1||MilliQ||total | ||
| + | |- | ||
| + | |3||1||0.5||5.5||60 | ||
| + | |} | ||
| + | at 37℃ for 2 hours | ||
| + | ====Electrophoresis==== | ||
| + | 写真お願いします。<br> | ||
| + | ①. pSB4K5<br> | ||
| + | ②. pSB4K5(Pst1)<br> | ||
| + | ③. pSB4K5(EcoR1)<br> | ||
| + | ④. pSB4K5(EcoR1, Pst1)<br> | ||
| + | ⑤. LacP-torA-GFP-DT<br> | ||
| + | ⑥. LacP-torA-GFP-DT(Xba1)<br> | ||
| + | ⑦. LacP-torA-GFP-DT(Pst1)<br> | ||
| + | ⑧. LacP-torA-GFP-DT(Xba1, Pst1)<br><br> | ||
| + | |||
| + | 写真お願いします。<br> | ||
| + | ①. 1kb ladder<br> | ||
| + | ②. J23101-Kil-DT(Xba1, Pst1)<br> | ||
| + | ③. J23101-Kil-DT(Xba1, Pst1)<br> | ||
| + | ④. J23101-Kil-DT(Xba1, Pst1)<br> | ||
| + | ⑤. J23101-Kil-DT(Xba1, Pst1)<br> | ||
| + | ⑥. 1kb ladder<br> | ||
| Line 4,706: | Line 3,251: | ||
{| class="wikitable" | {| class="wikitable" | ||
| - | !J23101-Kil-DT||BufferM||Xba1|| | + | !J23101-Kil-DT||BufferM||Xba1||Pst1||BSA||MilliQ||total |
|- | |- | ||
|15||3||1||1||3||7||30 | |15||3||1||1||3||7||30 | ||
| Line 4,718: | Line 3,263: | ||
{| class="wikitable" | {| class="wikitable" | ||
| - | !J23101-Kil-DT||BufferH|| | + | !J23101-Kil-DT||BufferH||Pst1||MilliQ||total |
|- | |- | ||
|3||1||0.5||5.5||10 | |3||1||0.5||5.5||10 | ||
| Line 4,730: | Line 3,275: | ||
====Ligation==== | ====Ligation==== | ||
{| class="wikitable" | {| class="wikitable" | ||
| - | !LacP-torA-GFP-DT( | + | !LacP-torA-GFP-DT(Xba1,Pst1) 9.7μg/mL||pSB4K5(EcoR1,Pst1) 9.6μg/mL||J23107-tatABCD-PsPA-DT(EcoR1,Spe1) 14.2μg/mL||Ligation High Ver.2||total |
|- | |- | ||
|7||2||7||8||24μL | |7||2||7||8||24μL | ||
|} | |} | ||
{| class="wikitable" | {| class="wikitable" | ||
| - | !pSB4K5( | + | !pSB4K5(EcoR1,Pst1) 9.6μg/mL||Ligation High Ver.2||MilliQ||total |
|- | |- | ||
|2||1||21||24μL | |2||1||21||24μL | ||
| Line 4,741: | Line 3,286: | ||
incubate at 37℃ for 2h | incubate at 37℃ for 2h | ||
====Electrophoresis==== | ====Electrophoresis==== | ||
| - | J23101-kil-DT( | + | J23101-kil-DT(Xba1,Pst1) (Xba1) (Pst1)<br> |
Lane1: 1kb ladder<br> | Lane1: 1kb ladder<br> | ||
Lane2: empty<br> | Lane2: empty<br> | ||
Lane3: plasmid<br> | Lane3: plasmid<br> | ||
| - | Lane4: | + | Lane4: Xba1<br> |
| - | Lane5: | + | Lane5: Pst1<br> |
| - | Lane6: | + | Lane6: Xba1,Pst1<br> |
====Liquid Culture==== | ====Liquid Culture==== | ||
We did liquid culture J23106-kil-DT(9/10, Amp)'s 6,7 at LB mediumAmp. | We did liquid culture J23106-kil-DT(9/10, Amp)'s 6,7 at LB mediumAmp. | ||
====Transformation==== | ====Transformation==== | ||
| - | + | J23107-tatABCD-pspA-DT-LacP-torA-GFP-DT<br> | |
pSB4K5 negative control<br> | pSB4K5 negative control<br> | ||
====Liquid culture==== | ====Liquid culture==== | ||
| - | + | J23107-tatABCD-pspA-DT-LacP-torA-GFP-DT:1~5<br> | |
====Colony PCR==== | ====Colony PCR==== | ||
| - | + | J23107-tatABCD-pspA-DT-LacP-torA-GFP-DT<br> | |
{|class="wikitable" | {|class="wikitable" | ||
!VF2||VR||Quick tag||MilliQ||total | !VF2||VR||Quick tag||MilliQ||total | ||
| Line 4,798: | Line 3,343: | ||
Lane2: empty<br> | Lane2: empty<br> | ||
Lane3: plasmid<br> | Lane3: plasmid<br> | ||
| - | Lane4: | + | Lane4: EcoR1<br> |
| - | Lane5: | + | Lane5: Xba1<br> |
| - | Lane6: | + | Lane6: Pst1<br> |
| - | Lane7: | + | Lane7: EcoR1,Pst1<br> |
| - | Lane8: | + | Lane8: Xba1,Pst1<br> |
====Electrophoresis==== | ====Electrophoresis==== | ||
| - | J23107-tatABCD-pspA-DT-LacP- | + | J23107-tatABCD-pspA-DT-LacP-torA-GFP-DT<br> |
Lane1: ladder<br> | Lane1: ladder<br> | ||
Lane2: 1<br> | Lane2: 1<br> | ||
| Line 4,813: | Line 3,358: | ||
====Ligation==== | ====Ligation==== | ||
{| class="wikitable" | {| class="wikitable" | ||
| - | !LacP-torA-GFP-DT( | + | !LacP-torA-GFP-DT(Xba1,Pst1) 9.7μg/mL||pSB4K5(EcoR1,Pst1) 9.6μg/mL||J23107-tatABCD-PsPA-DT(EcoR1,Spe1) 14.2μg/mL||Ligation High Ver.2||total |
|- | |- | ||
|7||4||7||9||27μL | |7||4||7||9||27μL | ||
| Line 4,819: | Line 3,364: | ||
(Negative control)<br> | (Negative control)<br> | ||
{| class="wikitable" | {| class="wikitable" | ||
| - | !pSB4K5( | + | !pSB4K5(EcoR1,Pst1) 9.6μg/mL||Ligation High Ver.2||MilliQ||total |
|- | |- | ||
|4||9||14||27μL | |4||9||14||27μL | ||
| Line 4,825: | Line 3,370: | ||
====Restriction enzyme processing==== | ====Restriction enzyme processing==== | ||
{|class="wikitable" | {|class="wikitable" | ||
| - | !torA-R9 101.8μg/mL||bufferH||MilliQ||EcoR1|| | + | !torA-R9 101.8μg/mL||bufferH||MilliQ||EcoR1||Pst1||total |
|- | |- | ||
|10||3||15||1||1||30 | |10||3||15||1||1||30 | ||
|} | |} | ||
{|class="wikitable" | {|class="wikitable" | ||
| - | !torA-R9 101.8μg/mL||bufferH||MilliQ||EcoR1|| | + | !torA-R9 101.8μg/mL||bufferH||MilliQ||EcoR1||Pst1||total |
|- | |- | ||
|3||1||5.5||0.5||0||10 | |3||1||5.5||0.5||0||10 | ||
|} | |} | ||
{|class="wikitable" | {|class="wikitable" | ||
| - | !torA-R9 101.8μg/mL||bufferH||MilliQ||EcoR1|| | + | !torA-R9 101.8μg/mL||bufferH||MilliQ||EcoR1||Pst1||total |
|- | |- | ||
|3||1||5.5||0||0.5||10 | |3||1||5.5||0||0.5||10 | ||
| Line 4,851: | Line 3,396: | ||
→35cycles<br><br> | →35cycles<br><br> | ||
====Electrophoresis==== | ====Electrophoresis==== | ||
| - | torA-R9,plasmid,( | + | torA-R9,plasmid,(EcoR1),(Pst1),(EcoR1,Pst1)<br> |
Lane1: 1kb ladder<br> | Lane1: 1kb ladder<br> | ||
Lane2: empty<br> | Lane2: empty<br> | ||
Lane3: plasmid<br> | Lane3: plasmid<br> | ||
| - | Lane4: | + | Lane4: EcoR1<br> |
| - | Lane5: | + | Lane5: Pst1<br> |
| - | Lane6: | + | Lane6: EcoR1,Pst1<br> |
Lane7: empty<br> | Lane7: empty<br> | ||
Lane8: 1kb ladder<br> | Lane8: 1kb ladder<br> | ||
====Liquid culture==== | ====Liquid culture==== | ||
| - | LacP- | + | LacP-tatA-GFP-DT (9/4) |
====PCR purification==== | ====PCR purification==== | ||
torA-R9 68.0μg/mL | torA-R9 68.0μg/mL | ||
| Line 4,867: | Line 3,412: | ||
==September 20== | ==September 20== | ||
====Gel extraction==== | ====Gel extraction==== | ||
| - | LacP-kil-DT ( | + | LacP-kil-DT (EcoR1,Pst1) (Xba1,Pst1)<br> |
====Restriction enzyme processing==== | ====Restriction enzyme processing==== | ||
{|class="wikitable" | {|class="wikitable" | ||
| Line 4,877: | Line 3,422: | ||
====Ligation==== | ====Ligation==== | ||
{| class="wikitable" | {| class="wikitable" | ||
| - | !LacP-kil-DT( | + | !LacP-kil-DT(Xba1,Pst1) 12.4μg/mL||pSB1C3(EcoR1,Pst1) 34.2μg/mL||J23107-tatABCD-PsPA-DT(EcoR1,Spe1) 14.2μg/mL||Ligation High Ver.2||total |
|- | |- | ||
|7||4||7||9||27μL | |7||4||7||9||27μL | ||
| Line 4,883: | Line 3,428: | ||
(Negative control)<br> | (Negative control)<br> | ||
{| class="wikitable" | {| class="wikitable" | ||
| - | !pSB1C3( | + | !pSB1C3(EcoR1,Pst1) ||Ligation High Ver.2||MilliQ||total |
|- | |- | ||
|4||9||14||27μL | |4||9||14||27μL | ||
| Line 4,900: | Line 3,445: | ||
at 37℃, 2 hours | at 37℃, 2 hours | ||
====Colony PCR==== | ====Colony PCR==== | ||
| - | We made | + | We made J23107-tatABCD-pspA-DT-LacP-torA-GFP-DT's 1~8 samples. |
{|class="wikitable" | {|class="wikitable" | ||
!Quick tag||tat f primer||VR||MilliQ||total | !Quick tag||tat f primer||VR||MilliQ||total | ||
| Line 4,912: | Line 3,457: | ||
→30cycles<br> | →30cycles<br> | ||
====Liquid culture==== | ====Liquid culture==== | ||
| - | We did liquid culture | + | We did liquid culture J23107-tatABCD-pspA-DT-LacP-torA-GFP-DT's 1~4 samples. |
====Electrophoresis==== | ====Electrophoresis==== | ||
J23701-tatABCD-pspA-DT-LacP-torA-GFP-DT | J23701-tatABCD-pspA-DT-LacP-torA-GFP-DT | ||
| Line 4,927: | Line 3,472: | ||
Lane11: empty<br> | Lane11: empty<br> | ||
Lane12: empty<br> | Lane12: empty<br> | ||
| - | torA-R9 ( | + | torA-R9 (EcoR1,Pst1): 31μg/mL, (280/280): 0.80, (280/230): 0.39<br> |
| - | pSB1C3( | + | pSB1C3(EcoR1,Pst1): 22μg/mL, (280/280):1.33, (280/230):0.6<br> |
| - | torA-R9 ( | + | torA-R9 (Spe1): 56μg/mL, (280/280): 1.11, (280/230): 0.69<br> |
| - | GFP-DT( | + | GFP-DT(Xba1): 45μg/mL, (280/280): 1.15, (280/230): 0.88<br> |
====Ligation==== | ====Ligation==== | ||
{| class="wikitable" | {| class="wikitable" | ||
| - | !torA-R9 ( | + | !torA-R9 (EcoR1,Pst1)||pSB1C3(EcoR1,Pst1)||Ligation High Ver.2||total |
|- | |- | ||
|8||2||5||15μL | |8||2||5||15μL | ||
|} | |} | ||
{| class="wikitable" | {| class="wikitable" | ||
| - | !torA-R9 ( | + | !torA-R9 (Spe1)||pSB1C3(Xba1)||Ligation High Ver.2||total |
|- | |- | ||
|3||3||3||9μL | |3||3||3||9μL | ||
| Line 4,944: | Line 3,489: | ||
at 16℃, 1hour | at 16℃, 1hour | ||
====Colony PCR==== | ====Colony PCR==== | ||
| - | J23107-tatABCD-pspA-DT- | + | J23107-tatABCD-pspA-DT-LacP-torA-GFP-DT (4k5)<br> |
numbering 9~15 | numbering 9~15 | ||
====Transformation==== | ====Transformation==== | ||
| - | J23107- | + | J23107-tatABCD-pspA-DT-LacP-torA-GFP-DT (4k5)<br> |
| - | J23107- | + | J23107-tatABCD-pspA-DT-LacP-torA-kil-DT(pSB1C3) <br> |
| - | Negative control ( | + | Negative control (pSB1C3)<br> |
| - | Negative control( | + | Negative control(pSB4K5)<br> |
| - | torA-R9( | + | torA-R9(pSB1C3)<br> |
====Liquid culture==== | ====Liquid culture==== | ||
| - | J23107- | + | J23107-tatABCD-pspA-DT-LacP-torA-GFP-DT: colony PCR(9~15)<br> |
{|class="wikitable" | {|class="wikitable" | ||
!2xQuick tag||VF||VR||MilliQ||total | !2xQuick tag||VF||VR||MilliQ||total | ||
| Line 4,976: | Line 3,521: | ||
→30cycles<br> | →30cycles<br> | ||
====Miniprep==== | ====Miniprep==== | ||
| - | + | J23701-tatABCD-pspA-DT-LacP-torA-GFP-DT:2,3<br> | |
2: 10.4μg/mL, (250/280): 1.45, (260/230): 0.95<br> | 2: 10.4μg/mL, (250/280): 1.45, (260/230): 0.95<br> | ||
3: 6.0μg/mL, (250/280): 1.32, (260/230): 0.79<br> | 3: 6.0μg/mL, (250/280): 1.32, (260/230): 0.79<br> | ||
| Line 4,984: | Line 3,529: | ||
==September 21== | ==September 21== | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
====Gel extraction==== | ====Gel extraction==== | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
torA-R9-GFP-DT(after PCR): 10.3μg/mL, (260/280): 1.28, (220/230): 0.49<br> | torA-R9-GFP-DT(after PCR): 10.3μg/mL, (260/280): 1.28, (220/230): 0.49<br> | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
====Electrophoresis==== | ====Electrophoresis==== | ||
| - | + | J23107-tatABCD-pspA-DT-LacP-torA-GFP-DT(kan): 9~15<br> | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | J23107- | + | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
====Electrophoresis==== | ====Electrophoresis==== | ||
| - | + | J23107-tatABCD-pspA-DT-LacP-torA-GFP-DT: 1,2<br> | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
Lane1: empty<br> | Lane1: empty<br> | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
Lane2: empty<br> | Lane2: empty<br> | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
Lane3: 2<br> | Lane3: 2<br> | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
Lane4: 3<br> | Lane4: 3<br> | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
Lane5: 1kb ladder<br> | Lane5: 1kb ladder<br> | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
====Restriction enzyme processing==== | ====Restriction enzyme processing==== | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
{|class="wikitable" | {|class="wikitable" | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
!LacP-kil-DT(3)||Xba1||Pst1||10xBSA||MilliQ||BufferM||total | !LacP-kil-DT(3)||Xba1||Pst1||10xBSA||MilliQ||BufferM||total | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
|- | |- | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
|15||1||1||3||7||3||30 | |15||1||1||3||7||3||30 | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
|} | |} | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
{|class="wikitable" | {|class="wikitable" | ||
| - | + | !LacP-torA-GFP-DP(9/5)||Xba1||Pst1||10xBSA||MilliQ||BufferM||total | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | !LacP-torA- | + | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
|- | |- | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
|15||1||1||3||7||3||30 | |15||1||1||3||7||3||30 | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
|} | |} | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
{|class="wikitable" | {|class="wikitable" | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
!LacP-kil-DT||Xba1||10xBSA||MilliQ||BufferM||total | !LacP-kil-DT||Xba1||10xBSA||MilliQ||BufferM||total | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
|- | |- | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
|2||0.5||1||5.5||1||10 | |2||0.5||1||5.5||1||10 | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
|} | |} | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
{|class="wikitable" | {|class="wikitable" | ||
| - | + | !LacP-torA-GFP-DP||Xba1||10xBSA||MilliQ||BufferM||total | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | !LacP-torA- | + | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
|- | |- | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
|2||0.5||1||5.5||1||10 | |2||0.5||1||5.5||1||10 | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
|} | |} | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
{|class="wikitable" | {|class="wikitable" | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
!LacP-kil-DT||Pst1||MilliQ||BufferH||total | !LacP-kil-DT||Pst1||MilliQ||BufferH||total | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
|- | |- | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
|2||0.5||6.5||1||10 | |2||0.5||6.5||1||10 | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
|} | |} | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
{|class="wikitable" | {|class="wikitable" | ||
| - | + | !LacP-torA-GFP-DP||Xba1||Pst1||MilliQ||BufferH||total | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | !LacP-torA- | + | |
| - | + | ||
|- | |- | ||
| - | |||
| - | |||
|2||0.5||1||6.5||1||10 | |2||0.5||1||6.5||1||10 | ||
| - | |||
|} | |} | ||
{|class="wikitable" | {|class="wikitable" | ||
| - | ! | + | !J23107-tatABCD-pspA-DT||Spe1||Pst1||BufferH||MilliQ||total |
| - | + | ||
|- | |- | ||
| - | |||
|20||1||1||4||14||40 | |20||1||1||4||14||40 | ||
|} | |} | ||
{|class="wikitable" | {|class="wikitable" | ||
| - | + | !J23107-tatABCD-pspA-DT||Spe1||BufferM||MilliQ||total | |
| - | + | ||
| - | ! | + | |
| - | + | ||
|- | |- | ||
| - | |||
|2||0.5||1||6.5||10 | |2||0.5||1||6.5||10 | ||
| - | |||
| - | |||
|} | |} | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
{|class="wikitable" | {|class="wikitable" | ||
| - | + | !J23107-tatABCD-pspA-DT||Pst1||BufferH||MilliQ||total | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | ! | + | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
|- | |- | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
|2||0.5||1||6.5||10 | |2||0.5||1||6.5||10 | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
|}<br> | |}<br> | ||
| - | + | at 37℃, 2hours | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | at 37℃, 2hours | + | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
==September 22== | ==September 22== | ||
| Line 5,517: | Line 3,593: | ||
torA-R9(Amp)2: 97.8μg/mL, (260/280): 1.67, (280/230): 1.72<br> | torA-R9(Amp)2: 97.8μg/mL, (260/280): 1.67, (280/230): 1.72<br> | ||
torA-R9(Amp)3: 104.2μg/mL, (260/280): 1.93, (280/230): 2.67<br> | torA-R9(Amp)3: 104.2μg/mL, (260/280): 1.93, (280/230): 2.67<br> | ||
| - | LacP- | + | LacP-torA-GFP-DT 6: 37.4μg/mL, (260/280): 2.24, (280/230): 3.27<br> |
| + | |||
====Electrophoresis==== | ====Electrophoresis==== | ||
| - | LaneA: | + | LaneA: J23107-tatABCD (9/6)<br> |
| - | LaneB: | + | LaneB: J23107-tatABCD-pspA-DT pSB3C5 (9/13)<br> |
| - | LaneC: | + | LaneC: J23107-tatABCD-pspA-DT 1C5 (9/15)<br> |
| - | LaneD: | + | LaneD: J23107-tatABCD-pspA-DT (9/15)<br> |
| - | LaneE: | + | LaneE: J23107-tatABCD-pspA-DT(pSB1C3) (9/18)<br> |
| - | LaneF: | + | LaneF: J23107-tatABCD-pspA-DT(pSB3C5) (9/18)<br> |
| - | LaneG: | + | LaneG: J23107-tatABCD-pspA-DT-LacP-torA-GFP-DT:2 (9/20)<br> |
| - | LaneH: | + | LaneH: J23107-tatABCD-pspA-DT-LacP-torA-GFP-DT:3 (9/20)<br> |
| - | LaneA~H are ( | + | LaneA~H are (EcoR1,Pst1)<br> |
====Restriction enzyme processing==== | ====Restriction enzyme processing==== | ||
{|class="wikitable" | {|class="wikitable" | ||
| - | ! | + | !J23107-tatABCD-pspA-DT-LacPtorA-GFP-DT:2||BufferH||Pst1||MilliQ||total |
|- | |- | ||
|15||3||1||12||30 | |15||3||1||12||30 | ||
|} | |} | ||
{|class="wikitable" | {|class="wikitable" | ||
| - | ! | + | !J23107-tatABCD-pspA-DT-LacP-torA-GFP-DT:3||BufferH||Pst1||MilliQ||total |
|- | |- | ||
|15||3||1||12||30 | |15||3||1||12||30 | ||
| Line 5,541: | Line 3,618: | ||
====Restriction enzyme processing==== | ====Restriction enzyme processing==== | ||
{|class="wikitable" | {|class="wikitable" | ||
| - | !LacP- | + | !LacP-torA-GFP-DT(37.4μg/mL)||BufferH||EcoR1||Pst1||total |
|- | |- | ||
|25||3||1||1||30 | |25||3||1||1||30 | ||
|}<br> | |}<br> | ||
====Electrophoresis==== | ====Electrophoresis==== | ||
| - | 1: | + | 1: J23107-tatABCD-pspA-DT-LacP-torA-GFP-DT:2<br> |
| - | 2: LacP-torA-GFP-DT ( | + | 2: LacP-torA-GFP-DT (EcoR1,Pst1) (9/22)<br> |
| - | 3: | + | 3: J23107-tatABCD-pspA-DT-LacP-torA-GFP-DT:3<br> |
==September 23== | ==September 23== | ||
| Line 5,562: | Line 3,639: | ||
*J23107-tatABCD-pspA-DT(pSB1C3)(Spe1, Pst1) | *J23107-tatABCD-pspA-DT(pSB1C3)(Spe1, Pst1) | ||
*LacP-torA-GFP-DT(Xba1, Pst1) | *LacP-torA-GFP-DT(Xba1, Pst1) | ||
| - | lane_1 1kb ladder | + | lane_1 1kb ladder <br> |
| - | lane_3 J23107-tatABCD-pspA-DT(pSB1C3)(Spe1, Pst1) | + | lane_3 J23107-tatABCD-pspA-DT(pSB1C3)(Spe1, Pst1) <br> |
| - | lane_5 LacP-torA-GFP-DT(Xba1, Pst1) | + | lane_5 LacP-torA-GFP-DT(Xba1, Pst1)<br> |
| + | |||
====Restriction==== | ====Restriction==== | ||
{|class="wikitable" | {|class="wikitable" | ||
| Line 5,649: | Line 3,727: | ||
==September 24== | ==September 24== | ||
| + | ====Miniprep==== | ||
| + | *J23107-tatABCD-pspA-DT-2 139.4μg/ml 1.69(260/280) 1.83(260/230) | ||
| + | *J23107-tatABCD-pspA-DT-3 134.7μg/ml 1.61(260/280) 1.69(260/230) | ||
| + | *J23107-tatABCD-pspA-DT-5 139.3μg/ml 1.55(260/280) 1.45(260/230) | ||
| + | *J23107-tatABCD-pspA-DT-6 138.5μg/ml 1.58(260/280) 1.40(260/230) | ||
| + | *LacP-torA-GFP-DT-4 | ||
| + | *LacP-torA-GFP-DT-7 | ||
| + | *T7-6His-GFP-DT-1 | ||
| + | *T7-6His-GFP-DT-2 | ||
| + | ====Colony PCR==== | ||
| + | LacP-GFP generater (1-3) | ||
| + | {|class="wikitable" | ||
| + | !2×Quick Taq||VF2||VR||MilliQ||Total | ||
| + | |- | ||
| + | |25||1||1||23||50 | ||
| + | |} | ||
| + | 94℃, 2min<br> | ||
| + | 94℃, 30sec<br> | ||
| + | 55℃, 30sec<br> | ||
| + | 68℃, 1min<br> | ||
| + | →30cycles<br><br> | ||
| + | |||
| + | 写真お願いします。<br> | ||
| + | ①-③, LacP-GFP generater (1-3) <br> | ||
| + | |||
| + | ====Liquid culture==== | ||
| + | *J23107-tatABCD-pspA-DT-LacP-torA-GFP-DT (1-4) | ||
| + | *J23107-tatABCD-pspA-DT-LacP-kil-DT (1-3) | ||
| + | ====Colony PCR==== | ||
| + | J23107-tatABCD-pspA-DT-LacP-torA-GFP-DT (1-4) <br> | ||
| + | J23107-tatABCD-pspA-DT-LacP-kil-DT (1-3) <br> | ||
| + | {|class="wikitable" | ||
| + | !2×Quick Taq||VF2||VR||MilliQ||Total | ||
| + | |- | ||
| + | |25||1||1||23||50 | ||
| + | |} | ||
| + | 94℃, 2min<br> | ||
| + | 94℃, 30sec<br> | ||
| + | 55℃, 30sec<br> | ||
| + | 68℃, 5min<br> | ||
| + | →30cycles<br><br> | ||
| + | |||
| + | ====Electrophoresis==== | ||
| + | 写真お願いします。<br> | ||
| + | lane1, 1kb ladder<br> | ||
| + | lane2-5, J23107-tatABCD-pspA-DT-LacP-torA-GFP-DT (1-4)<br> | ||
| + | lane6-8, J23107-tatABCD-pspA-DT-LacP-kil-DT (1-3)<br> | ||
| + | |||
| + | ==September 25== | ||
| + | ====Liquid culture==== | ||
| + | *J23107-tatABCD-pspA-DT-LacP-torA-GFP-DT(pSB4K5)-2 | ||
| + | ====Miniprep==== | ||
| + | J23107-tatABCD-pspA-DT-LacP-kil-DT(pSB1C3)-3: 26.2μg/mL, (260/280): 1.50, (280/230): 0.65<br> | ||
| + | J23107-tatABCD-pspA-DT-LacP-torA-GFP(pSB1C3)-3: 24.8μg/mL, (260/280): 1.56, (280/230): 0.76<br> | ||
| + | LacP-GFP generater-1: 42.0μg/mL, (260/280): 1.58, (280/230): 0.94<br> | ||
| + | ====Restriction enzyme processing==== | ||
| + | {|class="wikitable" | ||
| + | !J23107-tatABCD-pspA-DT-LacP-torA-GFP-DT(pSB1C3) (26.2ng/µL)!!EcoRl!!Spel!!buffer M!!MiliQ!!Total | ||
| + | |- | ||
| + | |15||1||1||3||10||30 | ||
| + | |} | ||
| + | {|class="wikitable" | ||
| + | !J23107-tatABCD-pspA-DT-LacP-torA-GFP-DT(pSB1C3) (26.2ng/µL)!!EcoRl!!buffer M!!MiliQ!!Total | ||
| + | |- | ||
| + | |2||0.5||1||6.5||10 | ||
| + | |} | ||
| + | {|class="wikitable" | ||
| + | !J23107-tatABCD-pspA-DT-LacP-torA-GFP-DT(pSB1C3) (26.2ng/µL)!!Spel!!buffer M!!MiliQ!!Total | ||
| + | |- | ||
| + | |2||0.5||1||6.5||10 | ||
| + | |} | ||
| + | {|class="wikitable" | ||
| + | !J23107-tatABCD-pspA-DT-LacP-kil-DT(pSB1C3) (24.8ng/µL)!!EcoRl!!Pstl!!buffer H!!MiliQ!!Total | ||
| + | |- | ||
| + | |15||1||1||3||10||30 | ||
| + | |} | ||
| + | {|class="wikitable" | ||
| + | !J23107-tatABCD-pspA-DT-LacP-kil-DT(pSB1C3) (24.8ng/µL)!!Pstl!!buffer H!!MiliQ!!Total | ||
| + | |- | ||
| + | |2||0.5||1||6.5||10 | ||
| + | |} | ||
| + | {|class="wikitable" | ||
| + | !J23107-tatABCD-pspA-DT-LacP-kil-DT(pSB1C3) (24.8ng/µL)!!EcoRl!!buffer H!!MiliQ!!Total | ||
| + | |- | ||
| + | |2||0.5||1||6.5||10 | ||
| + | |} | ||
| + | at 37℃ for 2 hours | ||
| + | ====Electrophoresis==== | ||
| + | 写真お願いします。<br> | ||
| + | lane1, 1kb ladder<br> | ||
| + | lane2, J23107-tatABCD-pspA-DT-LacP-torA-GFP-DT(pSB1C3)<br> | ||
| + | lane3, J23107-tatABCD-pspA-DT-LacP-torA-GFP-DT(pSB1C3) (EcoRl, Spel)<br> | ||
| + | lane4, J23107-tatABCD-pspA-DT-LacP-torA-GFP-DT(pSB1C3) (EcoRl)<br> | ||
| + | lane5, J23107-tatABCD-pspA-DT-LacP-torA-GFP-DT(pSB1C3) (Spel)<br> | ||
| + | lane6, J23107-tatABCD-pspA-DT-LacP-kil-DT(pSB1C3) | ||
| + | lane7, J23107-tatABCD-pspA-DT-LacP-kil-DT(pSB1C3) (EcoRl, Pstl)<br> | ||
| + | lane8, J23107-tatABCD-pspA-DT-LacP-kil-DT(pSB1C3) (EcoRl)<br> | ||
| + | lane9, J23107-tatABCD-pspA-DT-LacP-kil-DT(pSB1C3) (Pstl)<br> | ||
| + | lane10, LacP-torA-GFP-DT(EcoR1,Xba1)(9/15)<br> | ||
| + | lane11, LacP-torA-GFP-DT(EcoR1,Xba1)(9/17)<br> | ||
| + | lane12, 1kb ladder<br> | ||
| + | |||
| + | →The bands didn't appear, so we did one more electrophoresis.<br> | ||
| + | 写真お願いします。<br> | ||
| + | lane1, 1kb ladder<br> | ||
| + | lane2, LacP-torA-GFP-DT(EcoR1,Xba1)(9/17)<br> | ||
| + | lane3, J23107-tatABCD-pspA-DT-LacP-torA-GFP-DT(pSB1C3)<br> | ||
| + | lane4, J23107-tatABCD-pspA-DT-LacP-torA-GFP-DT(pSB1C3) (EcoRl, Spel)<br> | ||
| + | lane5, J23107-tatABCD-pspA-DT-LacP-torA-GFP-DT(pSB1C3) (EcoRl)<br> | ||
| + | lane6, J23107-tatABCD-pspA-DT-LacP-torA-GFP-DT(pSB1C3) (Spel)<br> | ||
| + | ====Liquid culture==== | ||
| + | *LacP-torA-GFP-DT(pSB4K5) (1-4) | ||
| + | ====Restriction enzyme processing==== | ||
| + | {|class="wikitable" | ||
| + | !J23107-tatABCD-pspA-DT-LacP-torA-GFP-DT(pSB1C3)!!EcoRl!!Spel!!buffer M!!MiliQ!!Total | ||
| + | |- | ||
| + | |15||1||1||3||10||30 | ||
| + | |} | ||
| + | {|class="wikitable" | ||
| + | !J23107-tatABCD-pspA-DT-LacP-kil-DT(pSB1C3)!!EcoRl!!Pstl!!buffer H!!MiliQ!!Total | ||
| + | |- | ||
| + | |15||1||1||3||10||30 | ||
| + | |} | ||
| + | at 37℃ for 1 hour | ||
| + | ====Electrophoresis==== | ||
| + | J23107-tatABCD-pspA-DT-LacP-torA-GFP-DT(pSB1C3) (EcoRl, Spel)<br> | ||
| + | J23107-tatABCD-pspA-DT-LacP-kil-DT(pSB1C3) (EcoRl, Pstl)<br> | ||
| + | →The bands didn't appear. | ||
| + | |||
| + | </div> | ||
{{Kyoto/footer}} | {{Kyoto/footer}} | ||
Latest revision as of 18:19, 25 September 2012
Secretion Notebook
February 7
Preculture
We started preculture at 12:10.
February 8
Culture
We start culturing with 300[mL] of LB medium.
| time | OD600 |
|---|---|
| 12:00 | start |
| 14:10 | 0.019 |
| 14:45 | 0.154 |
| 15:05 | 0.267 |
| 15:21 | 0.64 |
Making Competent Cell
We made competent cells.
Transformation
pGEM_TAP
LacP (BBa_R0011)
DT (BBa_B0015)
Making Culture Medium Plates
We made 200mL of ampicillin culture, kanamycin culture, and chloramphenicol culture.
Transformation
GFP(BBa_E0040) in pSB1A2
DT(BBa_B0015) in pSB1AK3
ara(BBa_I0500) in pSB2K3
LacP(BBa_R0011) in pSB1A2
February 9
transformation
BBa-E0040(GFP)(Mr.Fujita)
Liquid culture
DT.leap colony transformed on February 8
colony of competent cell made on February 8
February 10
Miniprep
DT2 43.9μg/ml(1.34 260/230 1.74 260/280)
LacP1 17.1μg/ml(1.54 260/280 0.83 260/230)
LacP2 18.0μg/ml(1.58 260/280 0.87 260/230)
transformation
B0040 1.4k PsB1A2 B0034 1.2M pSB1A2(from iGEM parts plate)
Competent cell
We did preculture for overnight. We put 1.5mL of preculture on 150mL of LB culture.
| time | OD600 |
|---|---|
| 11:45 | start |
| 13:30 | 0.048 |
| 14:30 | 0.168 |
| 15:03 | 0.256 |
| 15:20 | 0.405 |
| 15:35 | 0.459 |
| at last | 0.576 |
February 11
Checking Transformation efficiency
Conpetent cell's transformation efficiency is 1.3x10^4colonys/μg
February 13
Transformation
Const promoter J23110, J23109, J23100
| DNA | Competent cell | total |
|---|---|---|
| 1μL | 20 | 21 |
No colony was there on February 14
Liquid culture
LacP, DT, RBS(BBa_B0034),GFP
start at 20:00
in Plus grow with Ampicilin 3mL
February 14
Miniprep
concentration[μg/μL]
| LacP3 | 39.6 |
| LacP4 | 40.8 |
| LacP5 | 28.9 |
| RBS1 | 28.2 |
| RBS2 | 57.4 |
| RBS3 | 13.2 |
| DT3 | 69.7 |
| DT4 | 64.4 |
| DT5 | 61.5 |
| GFP1 | 64.0 |
| GFP2 | 50.5 |
| GFP3 | 66.0 |
Restriction
Const promoterJ23100
| DNA | Spe1 | Pst1 | Buffer2 | BSA | MilliQ | total |
|---|---|---|---|---|---|---|
| 20 | 0.5 | 0.5 | 3 | 0.5 | 5.5 | 30 |
at 37℃ for overnight
February 15
Making gel
1% Agarose gel
| Agarose | TAE |
|---|---|
| 1.6g | 160mL |
Electrophoresis
Gel No.1
| Restriction product | loading dye |
|---|---|
| 5μL | 1 |
The marker was 1kb ladder
It seemed that this restriction product was not cut.
Gel No.2
Lane1 : 1kb ladder
Lane2 : J23100 2μL + 6*Loading dye 1μL
Lane3 : J23100(Spe1,Pst1) 5μL
Lane4 : J23100(Spe1,Pst1) 2μL
- There were bands on lane_2 and we cannot identify these bands because the sample of lane_2 was not cut with any restriction enzyme.
- There must have been bands at 2100bp and 883bp on lane_3 and lane_4.
Testing whether restriction enzyme were deactivated or not
| DNA(DT) | restriction enzyme | Buffer | BSA | MilliQ | total |
|---|---|---|---|---|---|
| 10 | 0.5 | 3 | 0.5 | 16 | 30 |
at 37℃ for Oveernight
Restriction enzyme means Spe1(1,2) Pst1(1,2,3) in this time.
February 16
Electrophoresis
1. 1kb ladder
2. DT2
3. DT3
4. DT2 (Spe1-1)
5. DT2 (Spe1-2)
6. DT2 (Pst1-1)
7. DT2 (Pst1-2)
8. DT2 (Pst1-3)
9. DT3 (Pst1-4)
10. 1kb ladder
Pst1-1, Pst1-2, and Pst1-3 did not cut DNAs. They seemed to be deactivated.
Genomic PCR
| 10*Buffer for KOD Plus | 2mM dNTPs | 25mM MgSO4 | 10μM primer-f | 10μM primer-r | 158ng/μL Genomic DNA | KOD plus | MilliQ | total |
|---|---|---|---|---|---|---|---|---|
| 5 | 5 | 3 | 1.5 | 1.5 | 1 | 1 | 32 | 50 |
Electrophoresis
1. 1kb ladder
2. tatABCD (2.5kb)
3. TAMO reductase (2.7kb)
4. Negative control
We got bands of tatABCD but there were nonspecific amplification products.
We failed amplification of TAMO reductase.
Transformation
Constitutive promoter (BBa_J23107 , BBA_J23117)
High copy plasmid (pSB1AT3)
| DNA | competent cell |
|---|---|
| 1μL | 10μL |
February 17
PCR
We did PCR to amplify products of PCR that we had done yesterday but we could not amplify tatABCD.
Genomic PCR
| Buffer | dNTPs | MgSO4 | primer-f | primer-r | genomic DNA | KOD plus | MilliQ | total |
|---|---|---|---|---|---|---|---|---|
| 2.5 | 2.5 | 5 | 0.75 | 0.75 | 0.5 | 0.5 | 16 | 50 |
Predenature 94℃, 2min
Denature 98℃, 10sec
Annealing 57℃, 30sec
Extension 68℃, 2.5min
(30cycles)
Electrophoresis
1. 1kb ladder
2. TAMO reductase
3. Negative control
Restriction
| J23100 | Spe1 | Pst1 | Buffer2 | BSA | MilliQ | total |
|---|---|---|---|---|---|---|
| 10 | 0.5 | 0.5 | 3 | 0.5 | 15.5 | 30 |
Genomic PCR
| Buffer | dNTPs | MgSO4 | primer-f | primer-r | genomic DNA | KOD plus | MilliQ | total | |
|---|---|---|---|---|---|---|---|---|---|
| TMAO reductase | 2.5 | 2.5 | 3 | 0.75 | 0.75 | 0.5 | 0.5 | 15.5 | 25 |
| tatABCD | 2.5 | 2.5 | 2 | 0.75 | 0.75 | 0.5 | 0.5 | 15.5 | 25 |
| tatABCD | 2.5 | 2.5 | 2 | 0.75 | 0.75 | 5 | 0.5 | 10.5 | 25 |
Electrophoresis
1. 1kb ladder
2.3. TAMO reductase
4. tatABCD
5. tatABCD(10 times amount of genome)
Checking of restriction enzyme
| DT | enzyme | Buffer | BSA | MilliQ | total |
|---|---|---|---|---|---|
| 2 | 0.5 | 3 | 0.5 | 24 | 30 |
at 37℃ for overnight
We checked EcoR1 and Xba1.
February 18
Miniprep
| μg/mL | 260/280 | 230/260 | |
|---|---|---|---|
| JS3117-1 | 135 | 1.5 | 2.06 |
| JS3117-2 | 75 | 1.6 | 1.63 |
| JS3109-1 | 115 | 1.5 | 1.88 |
| JS3109-2 | 75 | 1.65 | 1.71 |
| pSB1AT3-1 | 70 | 1.66 | 1.83 |
| pSB1AT3-2 | 100 | 1.52 | 1.54 |
diluted to 25 times
Competent cell
We put 3mL of preculture product on yesterday onto 300mL of LB medium
| time | OD600 |
|---|---|
| 10:30 | start |
| 12:10 | 0.118 |
| 13:00 | 0.270 |
| 13:30 | 0.502 |
Transformation
| pSB1AT3-2 | competent cell | MilliQ | total |
|---|---|---|---|
| 0 | 20 | 10 | 30 |
| 2 | 20 | 8 | 30 |
| 10 | 20 | 0 | 30 |
- Results(on Feb. 19)
| pSB1AT3-2 | number of colony |
|---|---|
| 0 | 0 |
| 2 | 177 |
| 10 | 590 |
Transformation efficiency 7.4x10^4 colonys/μg
February 20
Restriction
sample 1
| DT plasmid | EcoR1 | Xba1 | Buffer | BSA | MilliQ | total |
|---|---|---|---|---|---|---|
| 7.5 | 0.5 | 0.5 | 3 | 0.5 | 18 | 30 |
sample2
| GFP plasmid | EcoR1 | Spe1 | Buffer | BSA | MilliQ | total |
|---|---|---|---|---|---|---|
| 10 | 0.5 | 0.5 | 3 | 0.5 | 15.5 | 30 |
Electrophoresis
- sample1
a. sample1 2μL + MilliQ 3μL + 6×Loading Dye 1μL
b. sample1 5μL + 6×Loading Dye 1μL
lane_1 1kb ladder
lane_2 a
lane_3 b
lane_4 a
lane_5 b
lane_6 a
lane_7 b
lane_8 1kb ladder
- sample2
c. sample2 2μL + MilliQ 3μL + 6×Loading Dye 1μL
d. sample2 5μL + 6×Loading Dye 1μL
lane_1 1kb ladder
lane_2 c
lane_3 d
lane_4 1kb ladder
PCR
| Buffer | dNTPs | MgSO4 | primer-f | primer-r | genomic DNA | KOD plus | MilliQ | total | |
|---|---|---|---|---|---|---|---|---|---|
| tatABCD1 | 2.5 | 2.5 | 1.5 | 0.75 | 0.75 | 0.5 | 0.5 | 16 | 25 |
| tatABCD2 | 2.5 | 2.5 | 1.5 | 0.75 | 0.75 | 0.5 | 0.5 | 16 | 25 |
| TMAO reductase1 | 2.5 | 2.5 | 2 | 0.75 | 0.75 | 0.5 | 0.5 | 15.5 | 25 |
| TMAO reductase2 | 2.5 | 2.5 | 2 | 0.75 | 0.75 | 5 | 0.5 | 10.5 | 25 |
Predenature 94℃ 2min
Denature 98℃ 10sec
Annealing 59℃ 30sec
Extension 68℃ 2.5min
→30cycles
| Buffer | dNTPs | MgSO4 | primer-f | primer-r | PCR products | genomic DNA | KOD plus | MilliQ | total | |
|---|---|---|---|---|---|---|---|---|---|---|
| tatABCD1 | 2.5 | 2.5 | 1.5 | 0.75 | 0.75 | 0 | 0.5 | 0.5 | 16 | 25 |
| tatABCD2 | 2.5 | 2.5 | 1.5 | 0.75 | 0.75 | 1 | 0 | 0.5 | 15.5 | 25 |
| TMAO reductase1 | 2.5 | 2.5 | 2 | 0.75 | 0.75 | 0 | 0 | 0.5 | 16 | 25 |
| TMAO reductase2 | 2.5 | 2.5 | 2 | 0.75 | 0.75 | 0 | 0 | 0.5 | 16 | 25 |
Predenature 94℃ 2min
Denature 98℃ 10sec
Annealing 59℃ 30sec
Extension 68℃ 2.5min
→35cycles
Electrophoresis
lane_1.1kb ladder
lane_2.tatABCD
lane_3.tatABCD
lane_4.TAMO1
lane_5.TAMO2
lane_6.1kb ladder
lane_1.1kb ladder
lane_2.tatABCD1
lane_3.tatABCD2
lane_4.TAMO
lane_5.TAMO
lane_6.1kb ladder
February 21
PCR (Advantage HF protocol)
| buffer | dNTPs | primer-f | primer-r | gDNA | PCR products | DW | polymerase | total | |
|---|---|---|---|---|---|---|---|---|---|
| tatABCD | 2.5 | 2.5 | 0.75 | 0.75 | 1 | 0 | 17 | 0.5 | 25 |
| TMAO | 2.5 | 2.5 | 0.75 | 0.75 | 0 | 1 | 17 | 0.5 | 25 |
Predenature 94℃ 1min
Denature 94℃ 30sec
Annealing 58℃ 30sec
Extension 68℃ 3min
→25cycles
Electrophoresis
1. 1kb ladder 2μL
2. tatABCD 5μL + 6×Loading Buffer 1μL
3. TAMO 5μL + 6×Loading Buffer 1μL
4. 1kb ladder 2μL
Liquid culture
pSB3C5-1,2
pSB4K5-1,2
February 22
Gel extraction
lane 1 of the gel 45.0μg/mL
PCR purification
product 38.2μg/mL
PCR
TMAO reductase
| Buffer | dNTPs | MgSO4 | prefix primer-f | suffix primer-r | product of gel extract | product of PCR purification(1ng/μL) | KOD plus | MilliQ | total | |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 5 | 5 | 4 | 1.5 | 1.5 | 0.5 | 0 | 1 | 32.5 | 50 |
| 2 | 5 | 5 | 4 | 1.5 | 1.5 | 1 | 0 | 1 | 32.5 | 50 |
| 3 | 5 | 5 | 4 | 1.5 | 1.5 | 0 | 0.5 | 1 | 32.5 | 50 |
| 4 | 5 | 5 | 4 | 1.5 | 1.5 | 0 | 1 | 1 | 32.5 | 50 |
94℃, 2min
98℃, 10sec
59℃, 30sec
68℃, 3min
→25cycles
Electrophoresis
1. 1kb ladder
2. TAMO1
3. TAMO2
4. TAMO3
5. TAMO4
6. constructive promoter 1-18C
7. constructive promoter Spe1
8. constructive promoter Pst1
9. 1kb ladder
PCR
| Buffer | dNTPs | MgSO4 | prefix primer-f | suffix primer-r | product of PCR purification(1ng/μL) | KOD plus | MilliQ | total | |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 5 | 5 | 3 | 1.5 | 1.5 | 0.5 | 1 | 32.5 | 50 |
| 2 | 5 | 5 | 3 | 1.5 | 1.5 | 1 | 1 | 32 | 50 |
| 3 | 5 | 5 | 3 | 1.5 | 1.5 | 2 | 1 | 31 | 50 |
| 4 | 5 | 5 | 3 | 1.5 | 1.5 | 3 | 1 | 30 | 50 |
| 5 | 5 | 5 | 3 | 1.5 | 1.5 | 10 | 1 | 29 | 50 |
| 6 | 5 | 5 | 3 | 1.5 | 1.5 | 0 | 1 | 33 | 50 |
94℃, 2min
98℃, 10sec
59℃, 30sec
68℃, 3min
→25cycles
Electrophoresis
Checking Dpn1
| Buffer | LacP(28.7ng/μL) | Dpn1 | MilliQ | total |
|---|---|---|---|---|
| 2 | 10 | 0.5 | 7.5 | 20 |
| 2 | 10 | 0 | 5 | 17 |
February 23
Colony PCR
tatABCD(2 samples)
| Buffer | dNTPs | MgSO4 | primer-f | primer-r | KOD plus | MilliQ | total |
|---|---|---|---|---|---|---|---|
| 5 | 5 | 3 | 1.5 | 1.5 | 1 | 33 | 50 |
Predenature 94℃ 1min
Denature 98℃ 10sec
Annealing 59℃ 30sec
Extension 68℃ 3min
→25cycles
Electrophoresis
1. 1kb ladder 2μL
2. tatABCD 1 5μL + 6×Loading Buffer 1μL
3. tatABCD 2 5μL + 6×Loading Buffer 1μL
4. 1kb ladder 2μL
Miniprep
pSB4K5 and pSB3C5
deluted it to 25 times and then measured it
pSB4K5 1 : 60.0 μg/ml 1.67(260/280) 1.98(260/230)
pSB4K5 2 : 55.0 μg/ml 1.49(260/280) 1.62(260/230)
pSB3C5-3 : 3.6 μg/ml 1.57(260/280) 3.00(260/230)
pSB3C5-4 : 1.3 μg/ml 1.44(260/280) 1.04(260/230)
Liquid culture
pSB3C5-3,4
Electrophoresis
1. 1kb ladder 2μL
2. pSB3C5-3 5μL, 6×loading dye 1μL
3. pSB3C5-4 5μL, 6×loading dye 1μL
4. 1kb ladder 2μL
February 27
Test of Dpn1
| Buffer2 | GFP2 | BSA | MilliQ | Dpn1 |
|---|---|---|---|---|
| 3 | 3 | 0.3 | 23 | 1 |
Colony PCR
| buffer | dNTPs | MgSO4 | Primer-f | Primer-r | MilliQ | KOD Plus | total | |
|---|---|---|---|---|---|---|---|---|
| Colony PCR(2 samples) | 5 | 5 | 3 | 1.5 | 1.5 | 33 | 1 | 50 |
| Negative control | 5 | 5 | 3 | 1.5 | 1.5 | 34 | 0 | 50 |
Predenature 94℃,2min
Denature 98℃,10sec
Annealing 59℃,30sec
Extension 68℃,3min
→25cycles
Electrophoresis
| sample | Loading Dye | MilliQ | |
|---|---|---|---|
| 1.1kb ladder | 2 | 0 | 0 |
| 2.product of PCR1 | 5 | 1 | 0 |
| 3.product of PCR2 | 5 | 1 | 0 |
| 4.product of PCR(Negative control) | 5 | 1 | 0 |
| 5.product of PCR(2/23) | 5 | 1 | 0 |
| 6.GFP2(DPN1) | 10 | 2 | 0 |
| 7.GFP2 | 3 | 2 | 7 |
| 8.1kb ladder | 2 | 0 | 0 |
Results of liquid culture
We measure this after dilute it to 10 times.
| pSB3C5-5 | pSB3C5-6 | pSB3C5-5(1% glucose) | pSB3C5-6(1% glucose) |
|---|---|---|---|
| 8.5[µg/ml] | -1.8 | -17.9 | -18.2 |
PCR
| buffer | dNTPs | MgSO4 | Primer-f(prefix) | Primer-r(suffix) | PCR purification product(1ng/µL) | MilliQ | KOD Plus | total | |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 5 | 5 | 3 | 1.5 | 1.5 | 0.2 | 32.8 | 1 | 50 |
| 2 | 5 | 5 | 3 | 1.5 | 1.5 | 0.5 | 32.5 | 1 | 50 |
- PCR purification product was that purification product(75ng/µL) of electrophoresis-3 deluted to 1ng/µL
Predenature 94℃,2min
Denature 98℃,10sec
Annealing 59℃,30sec
Extension 68℃,3min
→25cycles
February 28
Electrophoresis
1. 1kb ladder
2. PCR1 →Product of gel extraction : tatABCD with prefix and suffix 105[ng/µL]
3. PCR2
Restriction
| Buffer2 | plasmid(?) | enzyme | MilliQ | total |
|---|---|---|---|---|
| 2 | 2 | 0.2 | 15.8 | 20 |
incubate 1 hour at 37℃
Electrophoresis
1. 1kb ladder
2. Control (without enzymes)
3. EcoR1
4. Xba1 (crystallized)
5. Xba1 (with seal)
6. Spe1
7. Pst1
8. 1kb ladder
PCR and Electrophoresis
| Quick Taq Dye Mix | primer-f | primer-r | template | MilliQ | total |
|---|---|---|---|---|---|
| 25 | 1.0 | 1.0 | 0.5 | 22.5 | 50 |
Predenature 94℃,2min
Denature 94℃,30sec
Annealing 59℃,30sec
Extension 68℃,3min
→25cycles
Restriction
| BufferH | tatABCD | EcoR1 | Spe1 | MilliQ | total |
|---|---|---|---|---|---|
| 2 | 5 | 0.2 | 0.2 | 12.6 | 20 |
PCR purification
We eluted the product for 30µL MilliQ
Ligation
| Insert(tatABCD) | Vector(pSB1C3) | Ligation High | total |
|---|---|---|---|
| 10 | 1 | 5 | 16 |
4℃, overnight
February 29
Transformation
| tatABCD | competent cell | total |
|---|---|---|
| 1 | 10 | 11 |
Checking Restriction enzyme
| plasmid seems to be 1-18C promoter | Enzyme | Buffer | MilliQ | total |
|---|---|---|---|---|
| 2 | 0.2 | 2 | 15.8 | 20 |
Checking tatABCD
| tatABCD | Hind3 | Buffer | MilliQ | total |
|---|---|---|---|---|
| 5 | 0.2 | 2 | 12.8 | 20 |
PCR
| buffer | dNTPs | MgSO4 | Primer-f | Primer-r | ColE1(6.5ng/µL) / TMAO | MilliQ | KOD Plus Neo | total | |
|---|---|---|---|---|---|---|---|---|---|
| Kil | 2.5 | 2.5 | 1.5 | 0.75 | 0.75 | 0.5 | 16 | 0.5 | 25 |
| TMAO | 2.5 | 2.5 | 1.5 | 0.75 | 0.75 | 0.5 | 16 | 0.5 | 25 |
Predenature 94℃,2min
Denature 98℃,10sec
Annealing 60℃,30sec
Extension 68℃,3min
→30cycles
Electrophoresis
- evernoteに写真なし!至急求む
1. 1kb ladder
2. Kil (649bp)
3. TMAO (2720bp)
4. TMAO (Quick Taq)
5. tatABCD (Quick Taq)
6. tatABCD (Hind3)
7. 1kb ladder
March 1
PCR
- TMAO
Template is gDNA and product of colony PCR gel extraction
| Buffer | gNTPs | MgSO4 | Primer-f | Primer-r | KOD Plus Neo | Template gDNA | product of gel extraction | DW | total | |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2.5 | 2.5 | 1.5 | 0.75 | 0.75 | 0.5 | 0.5 | 0 | 16 | 25 |
| 2 | 2.5 | 2.5 | 1.5 | 0.75 | 0.75 | 0.5 | 0 | 2 | 14.5 | 25 |
94℃, 2min
98℃, 10sec
60℃, 30sec
68℃, 1.5min
→25cycles
- Kil
| Buffer | dNTPs | MgSO4 | primer-f | primer-r | colE1(6.5ng/µL) | KOD Plus Neo | MilliQ | total |
|---|---|---|---|---|---|---|---|---|
| 2.5 | 2.5 | 1.5 | 0.75 | 0.75 | 0.5 | 16 | 0.5 | 25 |
94℃, 2min
98℃, 10sec
61℃, 30sec
68℃, 1min
→20cycles
Electrophoresis
1. 1kb ladder
2. TMAO1 (gDNA)
3. TMAO2 (product of gel extraction)
4. Kil
PCR
| Buffer | dNTPs | MgSO4 | primer-f | primer-r | Product of Purification | KOD Plus Neo | MilliQ | total |
|---|---|---|---|---|---|---|---|---|
| 5 | 5 | 3 | 1.5 | 1.5 | 1 | 32 | 1 | 50 |
94℃, 2min
98℃, 10sec
61℃, 30sec
68℃, 30sec
20cycles
→Purification 230ng/µL
Restriction
| Kil | EcoR1 | Spe1 | BufferH | MilliQ | total |
|---|---|---|---|---|---|
| 5 | 0.2 | 0.2 | 2 | 12.6 | 20 |
incubate at 37℃, for 1.5 hours
PCR Purification
Ligation
| Kil | pSB1C3 | Ligation High | total |
|---|---|---|---|
| 5 | 1 | 3 | 9 |
at 4℃, for overnight
March 2
Ligation
| Kil | pSB1C3 | Ligation High Ver.2 | total |
|---|---|---|---|
| 5 | 1 | 3 | 9 |
| tatABCD | pSB1C3 | Ligation | total |
|---|---|---|---|
| 10 | 1 | 5 | 16 |
at 16℃ for 1 hour
Transformation
| Kil | Kil(3/1,Ligation) | tatABCD | competet cell | total |
|---|---|---|---|---|
| 1 | 0 | 0 | 10 | 11 |
| 0 | 1 | 0 | 10 | 11 |
| 0 | 0 | 1 | 10 | 11 |
PCR
| Quick Taq | primer-r | primer-f | template | MilliQ | total |
|---|---|---|---|---|---|
| 25 | 1 | 1 | 0.5 | 22.5 | 50 |
Electrophoresis
Restriction
| pSB3C5-5 | EcoR1 | Pst1 | BufferH | BSA | MilliQ | total |
|---|---|---|---|---|---|---|
| 20 | 0.5 | 0.5 | 3 | 0.5 | 5.5 | 30 |
at 37℃, for 2 hour
Electrophoresis
1. 1kb ladder 2µL
2. pSB3C5 5µL + 6×Loading Buffer 1µL
・product of gel extraction(about 2700bp)
-30.9µg/mL
Restriction
GFP1 ,2 ,3
| GFP | EcoR1 | Pst1 | Buffer | BSA | MilliQ | total |
|---|---|---|---|---|---|---|
| 10 | 0.5 | 0.5 | 3 | 0.5 | 15.5 | 30 |
at 37℃, for 2.5 hours
Restriction
| DT | EcoR1 | Xba1 | BufferM | BSA | MilliQ | total |
|---|---|---|---|---|---|---|
| 10 | 0.2 | 0.2 | 3 | 0.3 | 16.3 | 30 |
| Constitutive Promoter | Spe1 | Pst1 | BufferM | BSA | MilliQ | total |
|---|---|---|---|---|---|---|
| 15 | 0.2 | 0.2 | 3 | 0.3 | 11.3 | 30 |
at 37℃, 2 hours
- J23117-1:135ng/µL, J23107-1:115ng/µL
- DT3→PCR Purification
- Promoter→Gel Extraction
Checking TMAO
| something seems to be TMAO | Buffer2 | EcoR1 | MilliQ | total | 10 | 2 | 0.5 | 7.5 | 20 |
|---|
at 37℃, for 1 hour
Electrophoresis
1. 1kb ladder
2. GFP1 that had been cut by restriction enzyme
3. GFP2 that had been cut by restriction enzyme
4. GFP3 that had been cut by restriction enzyme
5. GFP1
6. GFP2
7. GFP3
8. TMAO (control)
9. TMAO (EcoR1)
10. DT (control)
11. DT (EcoR1, Xba1)
12. 1kb ladder
Checking tatABCD
| Quick Taq | primer-f | primer-r | MilliQ | total |
|---|---|---|---|---|
| 25 | 1 | 1 | 23 | 50 |
Electrophoresis
March 3
PCR
| template | buffer | dNTPs | MgSO4 | VF | VR | KOD plus | MilliQ | total | |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 1 | 5 | 5 | 3 | 1.5 | 1.5 | 1 | 32 | 50 |
| 2 | 2 | 5 | 5 | 3 | 1.5 | 1.5 | 1 | 31 | 50 |
94℃, 2min
98℃, 10sec
50℃, 30sec
68℃, 1min
→30cycles
Miniprep
March 4
Sequence of tatABCD
| Quick Taq | primer-f | promer-r sequence | template | MilliQ | total |
|---|---|---|---|---|---|
| 25 | 1 | 1 | 1 | 23 | 50 |
| Quick Taq | primer-f sequence | primer-r | template | MilliQ | total |
|---|---|---|---|---|---|
| 25 | 1 | 1 | 1 | 23 | 50 |
Colony PCR of TMAO
| buffer | dNTPs | NgSO4 | primer-f | primerr-r | KOD plus | MilliQ | total |
|---|---|---|---|---|---|---|---|
| 5 | 5 | 4 | 1.5 | 1.5 | 1 | 32 | 50 |
→ethanol precipitation
94℃, 2min
94℃, 30sec
55℃, 30sec
68℃, 2.5min
→25cycles
Electrophoresis
1. 1kb ladder
2. tatABCD1
3. tatABCD2
4. TMAO
5. 1kb ladder
Restriction
| TMAO | EcoR1 | BufferH | BSA | MilliQ | total |
|---|---|---|---|---|---|
| 10 | 0.2 | 3 | 0.3 | 16.5 | 30 |
| TMAO | Xba1 | Pst1 | BudderM | BSA | MilliQ | total |
|---|---|---|---|---|---|---|
| 10 | 0.2 | 0.2 | 3 | 0.3 | 16.3 | 30 |
at 37℃ for 1 hour
Electrophoresis
Transformation
| pSB1C3 | competent cell(made at 2/8) | total |
|---|---|---|
| 5 | 100 | 105 |
March 5
Restriction
| pSB1C3(Xba1, Spe1) | Pst1 | BufferH | BSA | MilliQ | total |
|---|---|---|---|---|---|
| 10 | 0.2 | 2 | 0.2 | 7.6 | 20 |
| pSB1C3(Xba1, Spe1) | EcoR1 | BufferH | BSA | MilliQ | total |
| 10 | 0.2 | 2 | 0.2 | 7.6 | 20 |
at 37℃ for 1 hour
→Then we did ethanol precipitation
Ligation
| Kil(EcoR1, Spe1) | pSB1C3(EcoR1) | Ligation High | total |
|---|---|---|---|
| 5 | 1 | 3 | 9 |
at 16℃ for 1 hour
Transformation
| Kil | competent cell | total |
|---|---|---|
| 1 | 10 | 11 |
We used commercially available competent cells in this time.
PCR
TMAO
| buffer | dNTPs | MgSO4 | Primer-f | Primer-r | Template | KOD plus | MilliQ | total |
|---|---|---|---|---|---|---|---|---|
| 5 | 5 | 3 | 1.5 | 1.5 | 1 | 1 | 32 | 50 |
Electrophoresis
(31)
Restriction
| LacP | pSB3C5 | EcoR1 | Pst1 | BufferH | BSA | MilliQ | total | |
|---|---|---|---|---|---|---|---|---|
| 1 | 20 | 0 | 0.5 | 0.5 | 3 | 0.5 | 5.5 | 30 |
| 2 | 0 | 20 | 0.5 | 0.5 | 3 | 0.5 | 5.5 | 30 |
at 37℃ for 1 hour
1→Ethanol precipitation 45.4µg/mL
2→Gel extraction 38.7µg/mL
Ligation
| LacP | pSB3C5 | Ligation High | total |
|---|---|---|---|
| 10 | 2 | 6 | 18 |
at 4℃ for overnight
Transformation
| LacP+pSB3C5 | competent cell | total |
|---|---|---|
| 1 | 10 | 11 |
on ice for 30 mins.
heat shock at 42℃ for 60secs
on ice for 2 mins.
After we incubate with 200µL of SOC culture for 1 hour, we did plating on LB culture with CP
Restriction
| GFP Plasmid | EcoR1 | Spe1 | Buffer2 | BSA | MilliQ | total |
|---|---|---|---|---|---|---|
| 10 | 0.5 | 0.5 | 3 | 0.5 | 15.5 | 30 |
at 37℃ for 2 hours
Electrophoresis
1. Ladder 2µL
2. GFP Plasmid (already restricted) 2µL + Loading Dye 2µL + MilliQ 8µL
3. Ladder 2µL
PCR
torA signal and pspA
pspAはコロニーPCR
| Buffer | dNTPs | MgSO4 | Primer-f | Primer-r | template(TMAO) | KOD plus | MilliQ | total |
|---|---|---|---|---|---|---|---|---|
| 2.5 | 2.5 | 1.5 | 0.75 | 0.75 | 0.5 | 0.5 | 16 | 25 |
94℃, 2min
98℃, 10sec
60℃, 30sec
68℃, 30sec
electrophoresis
1. 100bp Ladder
2. torA signal (272bp)
3. pspA (969bp)
4. 100bp Ladder
March 6
Restriction
| GFP | EcoR1 | Spe1 | BufferM | BSA | MilliQ | total |
|---|---|---|---|---|---|---|
| 12 | 0.5 | 0.5 | 3 | 0.5 | 13.5 | 30 |
We did incubate at 37℃ for 1.5hours.
And we did gel extraction on 3/7 and get 40.0μg/mL GFP.
PCR
| buffer | dNTPs | MgSO4 | Primer-f | Primer-r | template | KOD plus neo | MilliQ | total |
|---|---|---|---|---|---|---|---|---|
| 5 | 5 | 3 | 1.5 | 1.5 | 0.5 | 1 | 32.5 | 50 |
94℃ 2min
98℃ 10sec
60℃ 30sec
68℃ (torA 10sec / pspA 30sec) 25 cycles
We used product of PCR on 3/5 of torA and pspA as template.
Restriction
| Kil | EcoR1 | Spe1 | BufferM | BSA | MilliQ | total |
|---|---|---|---|---|---|---|
| 10 | 0.3 | 0.3 | 3 | 0.3 | 16.1 | 30 |
| DT | EcoR1 | Xba1 | Buffer2 | BSA | MilliQ | total |
|---|---|---|---|---|---|---|
| 10 | 0.5 | 0.5 | 3 | 0.5 | 15.5 | 30 |
at 37℃ for 2 hours
→ purification 37.7ng/μL
Ligation
| Kil | pSB1C3 | Ligation High Ver.2 | total |
|---|---|---|---|
| 4 | 2 | 3 | 9 |
- Kil : 350fmol
- pSB1C3 : 29fmol
at 16℃ for overnight
March 7
Electrophoresis
1. 1kb ladder 2µL
2. pspA (PCR product)2.5µL + Loading Dye 0.5µL
3. GFP 30µL + Loading Dye 6µL
4. 1kb ladder 2µL
- The GFP was gel extracted on 3/6 and concentrated by Vacuum in 150µg/mL
Ligation
| VectorDNA | GFP | Ligation High Ver.2 | total |
|---|---|---|---|
| 5 | 15 | 10 | 30 |
Restriction
| torA | EcoR1 | Spe1 | bufferM | BSA | MilliQ | total |
|---|---|---|---|---|---|---|
| 10 | 0.3 | 0.3 | 3 | 0.3 | 16.1 | 30 |
| pspA | EcoR1 | Spe1 | bufferM | BSA | MilliQ | total |
|---|---|---|---|---|---|---|
| 5 | 0.3 | 0.3 | 3 | 0.3 | 21.1 | 30 |
at 37℃ for 1.5 hours
Purification
torA→31.8ng/µL
pspA→49.3ng/µL
Ligation
| torA | pSB1C3 | Ligation High Ver.2 | total |
|---|---|---|---|
| 3 | 3 | 3 | 9 |
| pspA | pSB1C3 | Ligation High Ver.2 | total |
|---|---|---|---|
| 4 | 2 | 3 | 9 |
at 4℃, for overnight
- torA→31.8ng/µL×3µL=95.4ng=0.529pmol
- pSB1C3→19.4ng/µL×3µL=58.2ng=0.042pmol
- pspA→49.3ng/µL×4µL=197.2ng=0.308pmol
- pSB1C3→19.4ng/µL×2µL=38.8ng=0.029pmol
March 8
Restriction
| pSB4K5 | EcoR1 | Spe1 | BufferM | BSA | MilliQ | total |
|---|---|---|---|---|---|---|
| 20 | 0.2 | 0.2 | 3 | 0.2 | 6.4 | 30 |
at 37℃ for 1 hour.
→Purification : 36.6ng/µL
Ligation
| Kil | pSB4K5 | Ligation High Ver.2 | total |
|---|---|---|---|
| 10 | 1 | 5 | 16 |
at 4℃ for overnight
- Kil→37.7ng/µL×10µL=377ng=879fmol
- pSB4K5→36.6ng/µL×1µL=36.6ng=86fmol
Liquid culture
LacP + pSB3C5 -1, 2
Transformation
| torA | pspA | competent cell | total |
|---|---|---|---|
| 1 | 0 | 10 | 11 |
| 0 | 1 | 10 | 11 |
We use commercially available competent cells in this time.
March 9
Restriction
| tatABCD | Xba1 | Pst1 | BufferM | BSA | MilliQ | total | 10 | 0.2 | 0.2 | 3 | 0.3 | 16.3 | 30 |
|---|
at 37℃ for 1hour
→purification : 75.0μg/mL (1.11 260/280 , 0.81 260/230)
Miniprep
LacP + pSB3C5 1 80.5μg/mL (1.78 260/280 , 2.00 260/230)
LacP + pSB3C5 2 107.2μg/mL (1.83 260/280 , 1.90 260/230)
Colony PCR
| Quick Taq | VF | VR | MilliQ | total |
|---|---|---|---|---|
| 25 | 1 | 1 | 23 | 50 |
94℃ 2min
94℃ 30sec
55℃ 30sec
68℃ 6sec
25cycles
Ligation
| tatABCD | constP J23107 | Ligation High Ver.2 | total |
|---|---|---|---|
| 5 | 1 | 3 | 9 |
tatABCD : 227fmol
constP J23107 : 21fmol
Transformation
| pspA | torA | Kil | competent cell | |
|---|---|---|---|---|
| 1 | 1 | 0 | 0 | 10 |
| 2 | 0 | 1 | 0 | 10 |
| 3 | 0 | 0 | 1 | 10 |
Miniprep
4mL of plusgrow which had been cultured for overnight.
pSB4K5 : 80.5μg/mL
March 10
Screening PCR
| Quick Taq | VF | VR | MilliQ | total |
|---|---|---|---|---|
| 25 | 1 | 1 | 23 | 50 |
Then we did electrophoresis to confirm.
1.1kb ladder
2.kil (649bp)
3,4,5, pspA (969bp)
6,1kb ladder
1, 100bp ladder
2,3,4, torA signal
Restriction
| LacP-pSB3C5 | Spe1 | Pst1 | BufferM | BSA | MilliQ | total |
|---|---|---|---|---|---|---|
| 10 | 0.2 | 0.2 | 2 | 0.2 | 7.4 | 20 |
for 2.5 hours at 37℃
→purification 29.0ng/μL
| torA | Xba1 | Pst1 | BufferM | BSA | MilliQ | total |
|---|---|---|---|---|---|---|
| 10 | 0.2 | 0.2 | 2 | 0.2 | 7.4 | 20 |
for 2.5 hours at 37℃
→purification 91.8ng/μL
Ligation
| torA | LacP-pSB3C5 | Ligation High Ver.2 | total |
|---|---|---|---|
| 4 | 2 | 3 | 9 |
torA : 929fmol
LacP-pSB3C5 : 284fmol
for overnight at 4℃
March 11
Miniprep
We used 3μL of plus grow that we had cultured for overnight.
torA : 62.8ng/μL
Screening PCR
| Quick Taq | VF | VR | MilliQ | total |
|---|---|---|---|---|
| 25 | 1 | 1 | 23 | 50 |
Electrophoresis
1. 1kb ladder
2〜11. pspA (969bp)
12. 1kb ladder
The results were shown as photograph in the right.
It seemed that there were shorter sample than expected sample, so we did electrophoresis with pspA which was product of PCR and pspA which had already cut with EcoR1 and Spe1.
1.1kb ladder
2. pspA (PCR product)
3, pspA (Eco, Spe)
4〜6, pspA (colony PCR)
7,1kb ladder
The results were shown as photograph in the right.
March 12
Transformation
| DT(1ng/μL) | DT(0.1ng/μL) | Kil | LacP-torA | MilliQ | competent cell | total |
|---|---|---|---|---|---|---|
| 1 | 0 | 0 | 0 | 0 | 20 | 21 |
| 0 | 1 | 0 | 0 | 0 | 20 | 21 |
| 0 | 0 | 5 | 0 | 0 | 50 | 51 |
| 0 | 0 | 0 | 5 | 0 | 50 | 51 |
| 0 | 0 | 0 | 0 | 1 | 20 | 21 |
Restriction
| pspA | EcoR1 | Spe1 | BufferM | BSA | MilliQ | total |
|---|---|---|---|---|---|---|
| 10 | 0.5 | 0.5 | 3 | 0.3 | 15.7 | 30 |
at 37℃ for 4 hours
→ We did purification and got 48.3ng/μL pspA.
Ligation
| pspA | pSB1C3 | MilliQ | Ligation High Ver.2 | total |
|---|---|---|---|---|
| 4 | 2 | 0 | 3 | 9 |
| 2 | 2 | 0 | 2 | 6 |
| 0 | 2 | 2 | 2 | 6 |
March 13
Miniprep
pSB1C3 74.2µg/mL 1.65 (260/280) 1.31 (260/230)
March 14
Restriction
| pSB1C3 | EcoR1 | Spe1 | BufferM | BSA | MilliQ | total |
|---|---|---|---|---|---|---|
| 20 | 0.2 | 0.2 | 4 | 0.4 | 15.2 | 40 |
We did gel extraction and got 47.2ng/µL pSB1C3 but we did not cut out RFP.
Ligation
| torA | pSB1C3 | Ligation High Ver.2 | total |
|---|---|---|---|
| 4 | 2 | 3 | 9 |
| MilliQ | pSB1C3 | Ligation High Ver.2 | total |
| 4 | 2 | 3 | 9 |
at 16℃, for 1 hour
- torA : 0.707pmol
- pSB1C3 : 0.068pmol
Liquid culture
We cultured LacP-pSB3C5 1,2,3,4,5 (CP tolerance)on culture with Amp.
→Only 4 which did not be cultured succeeded.
March 15
Liquid culture
We cultured LacP-pSB3C5 6,7,8,9,10 on culture with ampicillin.
→6,8,9,10 were succeeded.
Restriction
| GFP | EcoR1 | Spe1 | Buffer2 | BSA | MilliQ | total |
|---|---|---|---|---|---|---|
| 10 | 0.5 | 0.5 | 3 | 0.5 | 15.5 | 30 |
| DT | EcoR1 | Xba1 | Buffer2 | BSA | MilliQ | total |
|---|---|---|---|---|---|---|
| 10 | 0.5 | 0.5 | 3 | 0.5 | 15.5 | 30 |
for 2 hours at 37℃.
Miniprep
pspA (pSB1C3) 40.5ng/µL
Ligation
| pspA | DT | Ligation High Ver.2 | total |
|---|---|---|---|
| 5 | 1.5 | 3 | 9.5 |
- pspA : 385fmol
- DT : 36fmol
Transformation
| J23107-tatABCD | DT (0.1ng/µL) | DT (0.01ng/µL) | pspA-DT | competent cells on 3/15 | total |
|---|---|---|---|---|---|
| 2 | 0 | 0 | 0 | 20 | 22 |
| 0 | 2 | 0 | 0 | 20 | 22 |
| 0 | 0 | 2 | 0 | 20 | 22 |
| 0 | 0 | 0 | 2 | 20 | 22 |
Screening PCR
Kil, pspA and torA
| Quick Taq | Primer-r | Primer-f | MilliQ | total | 25 | 1 | 1 | 23 | 50 |
|---|
March 16
Miniprep
torA (pSB1C3) 68.8ng/µL
Kil (pSB4K5) 92.7ng/µL
torA was red for some reason. We do not know why.
Colony PCR
| Quick Taq | Primer-r | Primer-f | MilliQ | total |
|---|---|---|---|---|
| 25 | 1 | 1 | 23 | 50 |
94℃, 2min
94℃, 30sec
55℃, 30sec
68℃, 6sec
→25cycles
Electrophoresis
The results were shown as photograph in the right.
Checking Transformation Efficiency
competent cells that were made on March 15.
DNA : 0.02ng → 668 colonies Transformation Efficiency : 3.3×10^7
DNA : 0.2ng → 1739 colonies Transformation Efficiency : 8.7×10^6
Restriction
| pSB1C3 | EcoR1 | Spe1 | BSA | BufferM | BufferH | MilliQ | total |
|---|---|---|---|---|---|---|---|
| 20 | 0.2 | 0.2 | 0.3 | 3 | 0 | 6.3 | 30 |
| 5 | 0.2 | 0 | 0.2 | 0 | 2 | 12.6 | 20 |
at 37℃, for 2 hours.
We did gel extraction for product with EcoR1, Spe1. We got 46.1ng/µL pSB1C3.
| Kil(pSB4K5) | EcoR1 | Pst1 | BSA | BufferH | MilliQ | total |
|---|---|---|---|---|---|---|
| 5 | 0.2 | 0.2 | 0.2 | 2 | 12.4 | 20 |
| 5 | 0.2 | 0 | 0.2 | 2 | 12.6 | 20 |
for overnight at 37℃.
We did this to confirm.
| pspA (pSB1C3) | EcoR1 | Pst1 | BSA | BufferH | MilliQ | total |
|---|---|---|---|---|---|---|
| 5 | 0.2 | 0.2 | 0.2 | 2 | 12.4 | 20 |
| 5 | 0.2 | 0 | 0.2 | 2 | 12.6 | 20 |
for overnight at 37℃.
We did this to confirm.
Ligation
| torA | pSB1C3 | Ligation High Ver.2 | total |
|---|---|---|---|
| 4 | 2 | 3 | 9 |
| MilliQ | pSB1C3 | Ligation High Ver.2 | total |
|---|---|---|---|
| 4 | 2 | 3 | 9 |
at 16℃, for overnight
- torA→767fmol
- pSB1C3→68fmol
March 17
Miniprep
J23107-tatABCD 72.7ng/µL
pspA-DT 50.5ng/µL
Checking the Insert
| J21037-tatABCD | EcoR1 | Pst1 | BSA | BufferH | MilliQ | total |
|---|---|---|---|---|---|---|
| 5 | 0.2 | 0.2 | 0.2 | 2 | 12.4 | 20 |
| 5 | 0.2 | 0 | 0.2 | 2 | 12.6 | 20 |
Success.
| pspA-DT | EcoR1 | Pst1 | BSA | BufferH | MilliQ | total |
|---|---|---|---|---|---|---|
| 5 | 0.2 | 0.2 | 0.2 | 2 | 12.4 | 20 |
| 5 | 0.2 | 0 | 0.2 | 2 | 12.6 | 20 |
Failed.
March 19
Restriction
| DT | EcoR1 | Xba1 | BSA | BufferM | MilliQ | total |
|---|---|---|---|---|---|---|
| 10 | 0.2 | 0.2 | 0.3 | 3 | 16.3 | 30 |
We did Gel extraction and got 17.2ng/µL of DT.
Ligation
| Kil | DT | Ligation High Ver.2 | total |
|---|---|---|---|
| 10 | 2 | 6 | 18 |
We did this for an hour at 16℃.
Restriction
| GFP | EcoR1 | Spe1 | BSA | BufferM | MilliQ | total |
|---|---|---|---|---|---|---|
| 10 | 0.5 | 0.5 | 0.5 | 3 | 15.5 | 30 |
We did this for 4 hours at 37℃
Ligation
| pspA | DT | Ligation High Ver.2 | total |
|---|---|---|---|
| 5 | 5 | 5 | 15 |
| pspA | pSB1C3 | Ligation High Ver.2 | total |
|---|---|---|---|
| 4 | 1 | 3 | 8 |
We did these for an hour at 16℃.
- pspA (5µL)→377fmol
- DT→39fmol
- pspA (4µL)→339fmol
- pSB1C3→34fmol
Transformation
pspA-DT, pspA (pSB1C3), torA (pSB1C3) and GFP-DT
March 20
Screaning PCR
| Quick Taq | Primer-R | Primer-F | MilliQ | total |
|---|---|---|---|---|
| 25 | 1 | 1 | 23 | 50 |
- pspA→○
- pspA-DT→○
- GFP-DT→○
- torA→×
- Kil-DT 6 of 8 sumples→○
| Quick Taq | VR | VF | MilliQ | total |
|---|---|---|---|---|
| 25 | 1 | 1 | 23 | 50 |
- pspA→○
- pspA-DT→×
Restriction
| torA | EcoR1 | Spe1 | BSA | BufferM | MilliQ | total |
|---|---|---|---|---|---|---|
| 10 | 0.3 | 0.3 | 0.3 | 3 | 16.1 | 30 |
| DT | EcoR1 | Xba1 | BSA | BufferM | MilliQ | total |
|---|---|---|---|---|---|---|
| 10 | 0.2 | 0.2 | 0.3 | 3 | 16.3 | 30 |
We did this for overnight at 37℃. And we did purification.
torA 34.2ng/µL
DT 28.0ng/µL
March 21
Miniprep
GFP-DT-1 : 77.7ng/µL
GFP-DT-2 : 67.6ng/µL
Restriction
| pSB1C3 | EcoR1 | Spe1 | BSA | BufferM | MilliQ | total |
|---|---|---|---|---|---|---|
| 10 | 0.2 | 0.2 | 0.3 | 3 | 16.3 | 30 |
| 5 | 0.2 | 0 | 0.2 | 2 | 12.6 | 20 |
We did this for 3 hours at 37℃, and then we did gel extraction. We got 25.1ng/µL pSB1C3
| GFP-DT | EcoR1 | Pst1 | Xba1 | BSA | BufferM | MilliQ | total |
|---|---|---|---|---|---|---|---|
| 5 | 0.2 | 0.2 | 0 | 0.2 | 2 | 12.4 | 20 |
| 5 | 0.2 | 0 | 0 | 0.2 | 2 | 12.6 | 20 |
| 10 | 0.2 | 0 | 0.2 | 0.3 | 3 | 16.3 | 30 |
We did this for 3 hours at 37℃, and then we did Purification. We got 30.7ng/µL GFP-DT.
Ligation
| torA | pSB1C3 | pspA | DT | GFP-DT | Ligation High Ver.2 | total | |
|---|---|---|---|---|---|---|---|
| 1 | 4 | 1 | 0 | 0 | 0 | 3 | 8 |
| 2 | 0 | 1 | 7 | 0 | 0 | 4 | 12 |
| 3 | 0 | 0 | 5 | 3 | 0 | 4 | 12 |
| 4 | 3 | 0 | 0 | 0 | 5 | 4 | 12 |
We did this for an hour at16℃.
March 22
PCR
We did PCR to amplify torA_signal that was product of PCR at March 5 with redesigned primers.
| Buffer | dNTPs | MgSO4 | Primer-F | Primer-R | Template | MilliQ | KOD plus neo | total |
|---|---|---|---|---|---|---|---|---|
| 5 | 5 | 3 | 1.5 | 1.5 | 0.5 | 32.5 | 1 | 50 |
94℃, 2min
98℃, 10sec
60℃, 30sec
68℃, 10sec
→30cycles
→Purification 110.7ng/µL
Restriction
| torA | EcoR1 | Spe1 | BSA | BufferM | MilliQ | total |
|---|---|---|---|---|---|---|
| 10 | 0.2 | 0.2 | 0.3 | 3 | 16.3 | 30 |
Ligation
| torA | pSSB1C3 | pspA | DT | GFP-DT | Ligation high | total | |
|---|---|---|---|---|---|---|---|
| 1 | 4 | 3 | 0 | 0 | 0 | 4 | 11 |
| 2 | 3 | 0 | 0 | 0 | 3 | 3 | 9 |
| 3 | 0 | 0 | 5 | 5 | 0 | 5 | 15 |
- torA (4µL)→512fmol
- pSB1C3→54fmol
- torA (3µL)→384fmol
- GFP-DT→36fmol
- pspA→377fmol
- DT→65fmol
March 23
Screening PCR
torA (pSB1C3), torA-GFP-DT and pspA-DT
| Quick Taq | VF | VR | MilliQ | total |
|---|---|---|---|---|
| 25 | 1 | 1 | 23 | 50 |
E.coli in liquid culture that had pspA(pSB1C3) also expressed RFP.
Restriction
| pspA | EcoR1 | Spe1 | BufferM | BSA | MilliQ | total |
|---|---|---|---|---|---|---|
| 5 | 0.3 | 0.3 | 3 | 0.3 | 21.1 | 30 |
And then we did ethanol precipitation
Ethanol precipitation
pspA 11.5ng/µL.
Miniprep
LacP+pSB3C5-8 77.9µg/mL
LacP+pSB3C5-10 69.0µg/mL
Kil+DT-4 58.0µg/mL
Kil+DT-7 15.2µg/mL
Restriction
| LacP+pSB3C5-8 | Spe1 | Pst1 | Buffer2 | BSA | MilliQ | total |
|---|---|---|---|---|---|---|
| 20 | 0.5 | 0.5 | 3 | 0.5 | 5.5 | 30 |
| Kil+DT-4 | Xba1 | Pst1 | Buffer2 | BSA | MilliQ | total |
|---|---|---|---|---|---|---|
| 20 | 0.5 | 0.5 | 3 | 0.5 | 5.5 | 30 |
at 37℃ for 1.5 hours
And then we did Gel extraction.
Gel Extraction
LacP+pSB3C5-8 40.4µg/mL
Kil+DT-4 26.8µg/mL
March 26
Miniprep
torA(pSB1C3) 18.5[ng/µL]
torA-GFP-DT 20.0[ng/µL]
Restriction
| torA(pSB1C3) | EcoR1 | Pst1 | BufferH | BSA | MilliQ | total |
|---|---|---|---|---|---|---|
| 5 | 0.2 | 0.2 | 2 | 0.2 | 12.4 | 20 |
| 5 | 0.2 | 0 | 2 | 0.2 | 12.6 | 20 |
| torA-GFP-DT | EcoR1 | Xba1 | Pst1 | BufferH | BufferM | BSA | MilliQ | total |
|---|---|---|---|---|---|---|---|---|
| 5 | 0.2 | 0 | 0.2 | 2 | 0 | 0.2 | 12.4 | 20 |
| 5 | 0.2 | 0 | 0 | 2 | 0 | 0.2 | 12.6 | 20 |
| 20 | 0 | 0.2 | 0.2 | 0 | 3 | 0.3 | 6.3 | 30 |
We did Gel extraction and then got ??? 28.7[ng/µL]
Ligation
| LacP (pSB3C5) | torA-GFP-DT | Ligation High Ver.2 | total |
|---|---|---|---|
| 1 | 5 | 3 | 9 |
- LacP : 22fmol
- torA-GFP-DT : 197fmol
| pspA | pSB1C3 | Ligation High Ver.2 | total |
|---|---|---|---|
| 10 | 1 | 5 | 16 |
| pspA | DT | Ligation High Ver.2 | total |
|---|---|---|---|
| 10 | 1 | 5 | 16 |
- pspA : 180fmol
- pSB1C3 : 18fmol
- DT : 16fmol
| LacP(pSB3C5) | Kil-DT | Ligation High Ver.2 | total |
|---|---|---|---|
| 1 | 5 | 3 | 9 |
for 2 hours at 16℃
Transformation
| LacP-Kil-DT | competent cell | total |
|---|---|---|
| 1 | 10 | 11 |
March 27
Miniprep
We retryed miniprep of torA(pSB1C3).
We got torA and its concentration was 39.3[ng/µL].
Transformation
| Name | Well | Sample | Competent Cells | Total | Plate | Colony |
|---|---|---|---|---|---|---|
| BBa_K117004 | 14J(2011 plate2) | 5 | 20 | ? | ? | ? |
We added 100[µL] of culture medium before we started culturing the E.coli.
Screening PCR
| Quick Taq | VF2 | VR | MilliQ | Total |
|---|---|---|---|---|
| 25 | 1 | 1 | 23 | 50 |
LacP-torA-GFP-DT 1, 3, 5, 6, 8, 9 was successful.
pspA-DT was failed.
E.coli that had pspA(pSB1C3) did not make any colony.
LacP-Kil-DT 1,2,3,4,5,6,7,8 was failed.
Liquid culture
LacP-torA-GFP-DT
July 23
Transformation
| DT(64.4ng/μl) | Competent cell | Plating in SOC medium | Total |
|---|---|---|---|
| 1 | 20 | 100 | 121 |
July 24
Plating in SOC medium
July 25
Transformation
- BBa_J23113 from iGEM Kit
- LacP from iGEM Kit
July 25
July 26
July 27
Restriction Enzyme Processing
| Kil +DT (44.0 ng/μl) | 10xBuffer2 | BSA | Xbal | MilliQ | Total |
|---|---|---|---|---|---|
| 13.6 | 3.0 | 0.5 | 0.5 | 11.9 | 30.0 |
| LacP(28.7ng/μl) | 10xBuffer2 | BSA | Spe1 | Pst1 | Total |
|---|---|---|---|---|---|
| 20.9 | 3.0 | 0.5 | 0.5 | 4.6 | 30.0 |
July 30
Ligation
| kil+DT(Xba1,Pst1) | LacP(Spe1,Pst1) | Ligation High | Total |
|---|---|---|---|
| 30 | 6 | 36 | 72 |
July 31
Restriction Enzyme Processing
| torA-GFP+DT | 10×M Buffer | BSA | MilliQ | Xba1 | Pst1 | Total |
|---|---|---|---|---|---|---|
| 2.5 | 3.0 | 0.5 | 23.4 | 0.3 | 0.3 | 30.0 |
Liquid Culture
J23113(backbone J61002)
August 1
Restriction Enzyme Processing
| LacP+kil+DT | BSA | 10×H Buffer | EcoR1 | Pst1 | MilliQ | Total |
|---|---|---|---|---|---|---|
| 5.0 | 0.5 | 3.0 | 0.5 | 0.5 | 20.5 | 30.0 |
| LacP middle copy | BSA | 10×H Buffer | EcoR1 | Pst1 | MilliQ | Total |
|---|---|---|---|---|---|---|
| 10.0 | 0.5 | 3.0 | 0.5 | 0.5 | 15.5 | 30.0 |
MIniprep
J23113(backbone J61002)
Liquid culture
J23113(backboneJ61002) Three test tubes of 4 mL LB medium with ampicillin 37℃ overnight
August 2
August 3
Restriction Enzyme processing
| pSB4K5 | BSA | 10×H Buffer | EcoR1 | Pst1 | MilliQ | Total |
|---|---|---|---|---|---|---|
| 8.0 | 0.5 | 3.0 | 0.5 | 0.5 | 17.5 | 30.0 |
| B0034 | BSA | 10×H Buffer | Spe1 | Pst1 | MilliQ | Total |
|---|---|---|---|---|---|---|
| 12.0 | 0.5 | 3.0 | 0.5 | 0.5 | 13.5 | 30.0 |
37℃ 2h
DNA purification
Ligation
| LacP+kil+DT | pSB4K5 | ligation high | Total |
|---|---|---|---|
| 15 | 15 | 15 | 45 |
Miniprep
- J23113(backbone J61002) 218.0μg/mL
- J23113(backbone J61002) 252.5μg/mL
- J23113(backbone J61002) 201.0μg/mL
- pSB3C5 45.0μg/ml 1.60 260/280 1.80 260/230
- pSB4C5 213.0μg/mL 1.77 260/280 4.40 260/230
August 4
August 5
August 6
August 7
August 8
August 9
August 10
Ligation
| B0034 Spe1 Pst1 | GFP+DT Xba1 Pst1 | ligation high | Total |
|---|---|---|---|
| 2 | 3 | 4 | 9 |
Transformation
- RBS+GFP+DT
- LacP+kil+DT
- RBS(for control)
- To put 2ng DNA in 20μL competent cell and leave it on ice for 30min
- Heat shock for 60s at 42℃
- To leave on ice for 2min
- To spread on ampicillin LB plate
August 11
August 12
August 13
Liquid culture
B0034 3mL ×3
- 37℃ overnight
August 14
Miniprep
- B0034 0μg/mL
- B0034 235.5μg/mL
- B0034 76.5μg/mL
Colony PCR
LacP+kil+DT ×5
| 2×Quick Taq | VF | VF | MilliQ | Total |
|---|---|---|---|---|
| 25 | 1 | 1 | 23 | 50 |
25cycles
pspA
| 10×Buffer for KOD-Plus-Ver2 | 2mM dNTPs | 25mM MgSO4 | Primer PsPA f-p | Primer PsPA r-s | KOD-Plus- | MilliQ | Total |
|---|---|---|---|---|---|---|---|
| 5 | 5 | 3 | 1.5 | 1.5 | 1 | 33 | 50 |
Electrophoresis
positive control(GFP+DT)
negative control(MilliQ)
① ~⑥ colony PCR LacP+kil+DT
次の写真
① 1kb ladder
② Positive control (GFP+DT)
③ Negative control (MilliQ)
④ ~⑨ LacP+kil+DT ①~⑥
⑩~⑫ pspA 10μL ,6×buffer 2μL
⑬ 1kb ladder
August 15
Colony PCR
pspA
| 2×Quick Taq | pspA r-s | pspA f-p | MilliQ | Total |
|---|---|---|---|---|
| 25 | 1 | 1 | 23 | 50 |
94℃, 2min
94℃, 30sec
56℃, 30sec
68℃, 1min
→35cycles
August 16
August 17
Colony PCR
pspA
| 10×Buffer for KOD-Plus-Ver2 | 2mM dNTPs | 25mM MgSO4 | Primer PsPA f | Primer PsPA r | KOD-Plus- | MilliQ | Total |
|---|---|---|---|---|---|---|---|
| 5 | 3 | 3 | 1.5 | 1.5 | 1 | 35 | 50 |
| 5 | 3 | 5 | 1.5 | 1.5 | 1 | 33 | 50 |
negative control(MilliQ 50μL)
94℃, 2min
94℃, 15sec
55℃, 30sec
68℃, 1min
→35cycles
Electrophoresis
①1kb ladder
②, ③ MgSO4 3μL, pspA
④, ⑤ MgSO4 5μL, pspA
⑥ negative control(MilliQ)
⑧ 1kb ladder
August 18
August 19
August 20
Restriction
| J23113(201.0ng/μL) | Xba1 | Pst1 | BufferM | BSA | MilliQ | total |
|---|---|---|---|---|---|---|
| 10 | 1 | 1 | 3 | 0.5 | 14.5 | 30 |
August 21
Colony PCR
pspA
| 10×Buffer | 2mM dNTPs | 25mM MgSO4 | Primer PsPA f | Primer PsPA r | KOD Plus Neo | MilliQ | Total |
|---|---|---|---|---|---|---|---|
| 5 | 3 | 5 | 1.5 | 1.5 | 1 | 33 | 50 |
| 5 | 3 | 7 | 1.5 | 1.5 | 1 | 31 | 50 |
| 5 | 3 | 10 | 1.5 | 1.5 | 1 | 28 | 50 |
94℃, 2min
98℃, 10sec
60℃, 30sec
68℃, 30sec
→25cycles
Restriction
| J23113(218ng/μL) | Spe1 | Pst1 | BufferH | BSA | MilliQ | total |
|---|---|---|---|---|---|---|
| 10 | 1 | 1 | 3 | 0.5 | 14.5 | 30 |
| torA-GFP-DT(20ng/μL) | Xba1 | Pst1 | BufferM | BSA | MilliQ | total |
|---|---|---|---|---|---|---|
| 5 | 0.6 | 0.6 | 3 | 0.5 | 20.3 | 30 |
Electrophoresis
① 1kb ladder
②, ③ torA-GFP-DT(Xba1,Pst1)
④, ⑤ J23113(Spe1,Pst1)
Transformation
- LacP-torA-GFP-DT(Backbone pSB3C5)
- BBa_K11704(Backbone pSB1A2)
Colony PCR
- pspA
| 10×Buffer | 2mM dNTPs | 25mM MgSO4 | Primer PsPA f | Primer PsPA r | KOD Plus Neo | MilliQ | Total |
|---|---|---|---|---|---|---|---|
| 5 | 5 | 3 | 1.5 | 1.5 | 1 | 33 | 50 |
| 10×Buffer | 2mM dNTPs | 25mM MgSO4 | Primer PsPA f-p | Primer PsPA r-s | KOD Plus Neo | MilliQ | Total |
|---|---|---|---|---|---|---|---|
| 5 | 5 | 3 | 1.5 | 1.5 | 1 | 33 | 50 |
- negative control for pspA
We did PCR without a template.
- GFP-DT
| 10×Buffer | 2mM dNTPs | 25mM MgSO4 | VF | VR | KOD Plus Neo | MilliQ | Total |
|---|---|---|---|---|---|---|---|
| 5 | 5 | 3 | 1.5 | 1.5 | 1 | 33 | 50 |
94℃, 2min
98℃, 10sec
60℃, 30sec
68℃, 30sec
Transformation
- LacP-torA-GFP-DT(Backbone pSB3C5)
- J23107-tatABCD
PCR
kil, torA-GFP
| 10×Buffer | 2mM dNTPs | 25mM MgSO4 | VF | VR | KOD Plus Neo | MilliQ | DNA | Total |
|---|---|---|---|---|---|---|---|---|
| 5 | 5 | 3 | 1.5 | 1.5 | 1 | 32 | 1 | 50 |
94℃, 2min
98℃, 10sec
60℃, 30sec
68℃, 30sec
→25cycles
August 22
Restriction
| pSB3C5 | EcoR1 | Pst1 | BufferH | BSA | MilliQ | total |
|---|---|---|---|---|---|---|
| 13.3 | 0.5 | 0.5 | 3 | 0.5 | 12.2 | 30 |
Gel extraction
pSB3C5(EcoR1, Pst1)
27.2μg/mL
Transformation(the second time)
- LacP-torA-GFP-DT(Backbone pSB3C5)
- J23107-tatABCD
Electrophoresis
写真お願いします。
See "August 21 -Colony PCR-"
1. 1kb ladder
2. pspA(Primer pspA f&r)
3. negative control for 2
4. GFP-DT
5. pspA(Primer pspA f-p&r-s)
6. pspA(Primer pspA f-p&r-s)
7. negative control for 5&6
PCR
LacP-GFP-DT(a kit of biological parts)
| 10×Buffer | 2mM dNTPs | 25mM MgSO4 | VF | VR | KOD Plus Neo | MilliQ | DNA | Total |
|---|---|---|---|---|---|---|---|---|
| 5 | 5 | 3 | 1.5 | 1.5 | 1 | 32 | 1 | 50 |
Colony PCR
pspA
| 10×Buffer | 2mM dNTPs | 25mM MgSO4 | Primer pspA f | Primer pspA r | KOD Plus Neo | MilliQ | Total |
|---|---|---|---|---|---|---|---|
| 5 | 5 | 3 | 1.5 | 1.5 | 1 | 33 | 50 |
| 10×Buffer | 2mM dNTPs | 25mM MgSO4 | Primer pspA f-p | Primer pspA r-s | KOD Plus Neo | MilliQ | Total |
|---|---|---|---|---|---|---|---|
| 5 | 5 | 3 | 1.5 | 1.5 | 1 | 33 | 50 |
94℃, 2min
98℃, 10sec
60℃, 30sec
68℃, 30sec
→30cycles
Electrophoresis
写真お願いします。
1. 1kb ladder
2. pspA(Primer pspA f&r)
3. pspA(Primer pspA f-p&r-s)
4. negative control for pspA(Primer pspA f&r)
5. negative control for pspA(Primer pspA f-p&r-s)
Transformation
Kil
August 23
Colony PCR
J23107-tatABCD
VF and VR were used for amplifying approximately 2800bp
| 10×Buffer | 2mM dNTPs | 25mM MgSO4 | VF | VR | KOD Plus Neo | MilliQ | DNA | Total |
|---|---|---|---|---|---|---|---|---|
| 5 | 5 | 3 | 1.5 | 1.5 | 1 | 32.5 | 0.5 | 50 |
94℃, 2min
98℃, 10sec
60℃, 30sec
68℃, 90sec
→25cycles
August 24
Purification of PCR products
pspA(Primer pspA f-p&r-s)
84.1μg/mL
→See "August 22 -Colony PCR-"
Restriction
| pspA | EcoR1 | Pst1 | BufferH | BSA | MilliQ | total |
|---|---|---|---|---|---|---|
| 20 | 0.5 | 0.5 | 3 | 0.5 | 5.5 | 30 |
| pspA | Xba1 | Pst1 | BufferM | BSA | MilliQ | total |
|---|---|---|---|---|---|---|
| 20 | 0.5 | 0.5 | 3 | 0.5 | 5.5 | 30 |
at 37℃ for 1h
Gel extraction
- pspA(EcoR1, Pst1) 30.7μg/mL
- pspA(Xba1, Pst1) 44.0μg/mL
Restriction
| pSB1C3(82.9μg/mL) | EcoR1 | Pst1 | BufferH | BSA | MilliQ | total |
|---|---|---|---|---|---|---|
| 20 | 0.5 | 0.5 | 3 | 0.5 | 5.5 | 30 |
→Gel extraction
Electrophoresis
1. 1kb ladder
2. J23107-tatABCD →See "August 23 -PCR-"
3. negative control for J23107-tatABCD
4. pSB1C3(EcoR1, Pst1)
Ligation
| pSB3C5(EcoR1, Pst1) | pspA(EcoR1, Pst1) | Ligation High Ver.2 | total |
|---|---|---|---|
| 1 | 5 | 3 | 9 |
16℃, overnight
August 25
August 26
August 27
Ligation
| pSB1C3(EcoR1, Pst1) | pspA(EcoR1, Pst1) | Ligation High Ver.2 | total |
|---|---|---|---|
| 1 | 5 | 3 | 9 |
16℃, overnight
Colony PCR
Kil (1-7)
| 10×Buffer | 2mM dNTPs | 25mM MgSO4 | VF | VR | KOD Plus Neo | MilliQ | Total |
|---|---|---|---|---|---|---|---|
| 5 | 5 | 3 | 1.5 | 1.5 | 1 | 33 | 50 |
94℃, 2min
98℃, 10sec
60℃, 30sec
68℃, 30sec
→30cycles
Purification of PCR products
torA-GFP →See "August 21 -PCR-"
Restriction
| LacP(Backbone:pSB3C5) | Spe1 | Pst1 | BufferH | BSA | MilliQ | total |
|---|---|---|---|---|---|---|
| 20 | 0.5 | 0.5 | 3 | 0.5 | 5.5 | 30 |
at 37℃ for 1h
→Gel extraction
| torA-GFP | Xba1 | Pst1 | BufferM | BSA | MilliQ | total |
|---|---|---|---|---|---|---|
| 20 | 0.5 | 0.5 | 3 | 0.5 | 5.5 | 30 |
at 37℃ for 1h
→Purification
Purification of PCR products
J23107-tatABCD
42.0μg/mL
→See "August 23 -PCR-"
Restriction
| J23107-tatABCD | Spe1 | EcoR1 | BufferM | BSA | MilliQ | total |
|---|---|---|---|---|---|---|
| 20 | 0.5 | 0.5 | 3 | 0.5 | 5.5 | 30 |
August 28
Colony PCR
Kil (1-16)
| 10×Buffer | 2mM dNTPs | 25mM MgSO4 | VF | VR | KOD Plus Neo | MilliQ | Total |
|---|---|---|---|---|---|---|---|
| 5 | 5 | 3 | 1.5 | 1.5 | 1 | 33 | 50 |
Transformation
- pspA-pSB1C3
- pspA-pSB3C5
Liquid culture
- Kil-3 →Miniprep 100.4μg/mL
- Kil-6
- Kil-7 →Miniprep 77.7μg/mL
- LacP-torA-GFP-1 →Miniprep 88.3μg/mL
- LacP-torA-GFP-2 →Miniprep 85.0μg/mL
- LacP-torA-GFP-3 →Miniprep +++
August 29
Kil assay
evernoteに結果のグラフがあります。
Liquid culture
- pspA-pSB1C3(1-3)
- pspA-pSB3C5(1-3)
Restriction
| LacP-Kil-DT(134.7ng/μL) | EcoR1 | Spe1 | BufferM | MilliQ | total |
|---|---|---|---|---|---|
| 10 | 1 | 1 | 3 | 15 | 30 |
37℃, overnight
Colony PCR
- LacP-torA-GFP-DT (1-5)
- pspA-pSB1C3 (1-5)
- pspA-pSB3C5 (1-6)
| 10×Buffer | 2mM dNTPs | 25mM MgSO4 | VF | VR | KOD Plus Neo | MilliQ | Total |
|---|---|---|---|---|---|---|---|
| 5 | 5 | 3 | 1.5 | 1.5 | 1 | 33 | 50 |
94℃, 2min
98℃, 10sec
60℃, 30sec
68℃, 40sec
→30cycles
Gel extraction
J23107-tatABCD(EcoR1, Pst1) -5.9μg/mL
August 30
Ligation
| J23107-tatABCD(EcoR1, Spe1)22.2μg/mL | DT(EcoR1, Xba1)37.5μg/mL | Ligation High Ver.2 | total |
|---|---|---|---|
| 10 | 1 | 9 | 20 |
at 16℃ for 1h
Transformation
J23107-tatABCD-DT (Amp resistance)
Electrophoresis
1. LacP-torA-GFP-DT-1
2. LacP-torA-GFP-DT-2
3. LacP-torA-GFP-DT-3
4. LacP-torA-GFP-DT-4
5. LacP-torA-GFP-DT-5
6. pspA-pSB1C3-1
7. pspA-pSB1C3-2
8. pspA-pSB1C3-3
9. pspA-pSB1C3-4
10.pspA-pSB1C3-5
11.pspA-pSB3C5-1
12.pspA-pSB3C5-2
13.pspA-pSB3C5-3
14.pspA-pSB3C5-4
15.pspA-pSB3C5-5
16.pspA-pSB3C5-6
Miniprep
- pspA-pSB3C5-1 176μg/mL
- pspA-pSB3C5-2 214μg/mL
- pspA-pSB3C5-3 461μg/mL
- pspA-pSB1C3-2 79μg/mL
Colony PCR
LacP-torA-GFP-DT (6-13)
| 2×Quick Taq | VF | VR | MilliQ | Total |
|---|---|---|---|---|
| 25 | 1 | 1 | 23 | 50 |
94℃, 2min
94℃, 30sec
56℃, 30sec
68℃, 1min
→30cycles
Restriction
| pspA-pSB1C3 | EcoR1 | Spe1 | BufferM | BSA | MilliQ | total |
|---|---|---|---|---|---|---|
| 15 | 0.5 | 0.5 | 5 | 0.5 | 28.5 | 50 |
at 37℃ for 1h
Electrophoresis
1. 1kb ladder
3. pspA-pSB1C3(EcoR1, Spe1)
August 31
Gel extraction
pspA-pSB1C3(EcoR1, Spe1) 8.0μg/mL
Purification
LacP-GFP-DT -0.7μg/mL
Ligation
| pspA(EcoR1, Spe1) | DT(EcoR1, Xba1) | Ligation High Ver.2 | total |
|---|---|---|---|
| 25 | 1 | 13 | 39 |
at 16℃ for 1h
Transformation
pspA-DT (Amp resistance)
September 1
September 2
Colony PCR
J23107-tatABCD-DT (1-8)
| 2×Quick Taq | VF | VR | MilliQ | Total |
|---|---|---|---|---|
| 25 | 1 | 1 | 23 | 50 |
94℃, 2min
94℃, 30sec
55℃, 30sec
68℃, 3min
→25cycles
pspA-DT (2-8)
| 2×Quick Taq | VF | VR | MilliQ | Total |
|---|---|---|---|---|
| 25 | 1 | 1 | 23 | 50 |
Extension, 10sec
→25cycles
Restriction
| LacP-torA-GFP-DT | EcoR1 | Spe1 | BufferH | MilliQ | total |
|---|---|---|---|---|---|
| 10 | 1 | 1 | 2 | 6 | 20 |
at 4℃ for overnight
Electrophoresis
1. 1kb ladder
3. J23107-tatABCD-DT-1
4. J23107-tatABCD-DT-3
5. J23107-tatABCD-DT-4
6. J23107-tatABCD-DT-5
7. J23107-tatABCD-DT-6
8. J23107-tatABCD-DT-7
9. J23107-tatABCD-DT-8
1. 1kb ladder
3. J23107-tatABCD-DT-1
4. J23107-tatABCD-DT-3
5. J23107-tatABCD-DT-4
6. J23107-tatABCD-DT-5
7. J23107-tatABCD-DT-6
8. J23107-tatABCD-DT-7
9. J23107-tatABCD-DT-8
11. LacP-pSB3C5(Spe1, Pst1)
1. 1kb ladder
3. pspA-DT-2
4. pspA-DT-3
5. pspA-DT-4
6. pspA-DT-5
7. pspA-DT-6
8. pspA-DT-7
9. pspA-DT-8
September 3
PCR
tatABCD-DT
| 10×Buffer | 2mM dNTPs | 25mM MgSO4 | VF | VR | KOD Plus Neo | MilliQ | DNA | Total |
|---|---|---|---|---|---|---|---|---|
| 5 | 5 | 3 | 1.5 | 1.5 | 1 | 32 | 1 | 50 |
Gel extraction
LacP-torA-GFP-DT →See "September 5 -Gel extraction-"
Restriction
| torA-GFP-DT | Xba1 | Pst1 | BufferM | MilliQ | total |
|---|---|---|---|---|---|
| 20 | 2 | 2 | 4 | 12 | 40 |
Purification
J23107-tatABCD 29.6μg/mL 1.56(260/280) 1.60(260/230)
Electrophoresis
1. 1kb ladder
2. J23107-tatABCD
Liquid culture
- LacP-kil-DT(1)
- pspA-DT(4, 7)
PCR
J23107-tatABCD
| 10×Buffer | 2mM dNTPs | 25mM MgSO4 | VF | VR | KOD Plus Neo | MilliQ | DNA | Total |
|---|---|---|---|---|---|---|---|---|
| 5 | 5 | 3 | 1.5 | 1.5 | 1 | 29 | 4 | 50 |
94℃, 2min
98℃, 10sec
60℃, 30sec
68℃, 10sec
→30cycles
Gel extraction
LacP-torA-GFP-DT 31μg/mL 1.28(260/280) 0.49(260/230) LacP-torA-GFP-DT 8μg/mL 1.32(260/280) 0.83(260/230)
Transformation
J23107-tatABCD (Amp resistance)
J23113-kil-DT (Amp resistance)
J23100-kil-DT (CP resistance)
J23101-kil-DT (CP resistance)
J23106-kil-DT (CP resistance)
J23115-kil-DT (CP resistance)→We didn't transform because of lack of culture medium.
J23105-kil-DT (CP resistance)→We didn't transform because of lack of culture medium.
Electrophoresis
1. 1kb ladder
2-7. LacP-torA-GFP-DT
Colony PCR
LacP-torA-GFP-DT (14-21) J23113-kil-DT (Amp resistance, 1-4) J23113-kil-DT (CP resistance, 1-4)
| 2×Quick Taq | primer-f | primer-r | MilliQ | Total |
|---|---|---|---|---|
| 25 | 1 | 1 | 23 | 50 |
94℃, 2min
94℃, 30sec
55℃, 30sec
68℃, 1min
→25cycles
September 4
Liquid culture
- LacP-kil-DT(4, 5)(Amp resistance)
- pspA-DT(3, 6, 7)(Amp resistance)
PCR
We did PCR 10 more cycles to amplify products of PCR that we had done yesterday because we could not amplify J23107-tatABCD on September 3.
94℃, 2min
98℃, 10sec
60℃, 30sec
68℃, 1.5min
→30cycles
Electrophoresis
1-8. LacP-torA-GFP-DT
1-4. J23113-kil-DT (Amp resistance)
5-8. J23113-kil-DT (CP resistance)
Liquid culture
- LacP-torA-GFP-DT
- J23113-kil-DT
- LacP-kil-DT(with IPTG)
- LacP-kil-DT(without IPTG)
After 20 hour, we quantified OD600.
| plasmid | OD600 |
|---|---|
| LacP-kil-DT(with IPTG) | 2.116 |
| LacP-kil-DT(without IPTG) | 2.320 |
We cultured CP tolerance plasmid and give an antibiotic(Amp or Kan or none) after 2 hours. We quantify OD600.
| time | 1 | 2 | 3 |
|---|---|---|---|
| 2hours | 0.131 | 0.100 | 0.606 |
| add an antibiotic | Amp | Kan | none |
| 7hours | 0.491 | 0.988 | 2.634 |
| 19hours | 2.253 | 0.815 | 2.742 |
September 5
Colony PCR
J23107-tatABCD (1-7)
| buffer | 2mM dNTPs | 25mM MgSO4 | primer-f | primer-r | KOD Plus Neo | DW | Total |
|---|---|---|---|---|---|---|---|
| 5 | 5 | 3 | 1.5 | 1.5 | 1 | 33 | 50 |
94℃, 2min
98℃, 10sec
60℃, 30sec
68℃, 1.25min
→30cycles
Gel extraction
by Kato
See "September 3 -Gel extraction-"
LacP-torA-GFP-DT 15.9μg/mL 1.29(260/280) 0.38(260/230)
LacP-torA-GFP-DT 17.2μg/mL 1.23(260/280) 0.48(260/230)
Miniprep
- J23113-kil-DT 85.7μg/mL 1.76(260/280) 1.97(260/230)
- LacP-torA-GFP-DT 126.6μg/mL 1.81(260/280) 2.09(260/230)
- LacP-kil-DT-4 62.5μg/mL 1.79(260/280) 1.99(260/230)
- LacP-kil-DT-5 56.0μg/mL 1.79(260/280) 2.01(260/230)
- pspA-DT-3 322μg/mL 1.45(260/280) 1.42(260/230)
- pspA-DT-6 147.6μg/mL 1.25(260/280) 1.37(260/230)
- pspA-DT-7 145.5μg/mL 1.24(260/280) 1.27(260/230)
Restriction
| pspA-DT-6 | Xba1 | Pst1 | BufferM | BSA | MilliQ | total |
|---|---|---|---|---|---|---|
| 20 | 0.5 | 0.5 | 3 | 0.5 | 5.5 | 30 |
at 37℃ for 1h
Electrophoresis
J23107-tatABCD (1-7)
Colony PCR
J23107-tatABCD (8-23)
| 2×Quick Taq | VF2 | VR | MilliQ | Total |
|---|---|---|---|---|
| 25 | 1 | 1 | 23 | 50 |
94℃, 2min
94℃, 30sec
55℃, 30sec
68℃, 1.5min
→30cycles
Gel extraction
pspA-DT 10.2μg/mL 1.42(260/280) 0.76(260/230)
Ligation
| pspA-DT(Xba1, Pst1) | LacP(pSB3C5)(Spe1, Pst1) | Ligation High Ver.2 | total |
|---|---|---|---|
| 5 | 1 | 3 | 9 |
at 16℃
Electrophoresis
LacP-torA-GFP-DT(8-15)
LacP-torA-GFP-DT(9-23)
Ligation
| LacP-torA-GFP-DT(EcoR1, Spe1) | DT 17.2μg/mL(Xba1, Pst1) | Ligation High Ver.2 | total |
|---|---|---|---|
| 15 | 25 | 20 | 60 |
Liquid culture
- J23107-tatABCD-8
- J23107-tatABCD-20
- LacP-torA-GFP-DT-21
September 6
Miniprep
- J23107-tatABCD-8 82.4μg/mL 2.06(260/280) 2.95(260/230)
- J23107-tatABCD-20 102.9μg/mL 2.04(260/280) 2.94(260/230)
Restriction
| J23107-tatABCD 102.9μg/mL | Spe1 | Pst1 | BufferH | BSA | MilliQ | total |
|---|---|---|---|---|---|---|
| 10 | 0.5 | 0.5 | 3 | 0.5 | 15.5 | 30 |
at 37℃ for 1h
Transformation
LacP-pspA-DT(pSB3C5)
| DNA | Competent cell | total |
|---|---|---|
| 2 | 20 | 22 |
Gel extraction
J23107-tatABCD(Spe1, Pst1) →We failed gel extraction because we mistook steps of experiment.
Electrophoresis
LacP-torA-GFP-DT-pspA-DT
Restriction
by Terasaka
| J23107-tatABCD | Spe1 | Pst1 | BufferH | BSA | MilliQ | total |
|---|---|---|---|---|---|---|
| 10 | 0.5 | 0.5 | 3 | 0.5 | 15.5 | 30 |
Gel extraction
J23107-tatABCD(Spe1, Pst1) →See "September 7 -Gel extraction-"
September 7
Liquid culture
- LacP-torA-GFP-DT-1 (CP resistance)
- LacP-torA-GFP-DT-21 (CP resistance)
preculture for 1 hour
Colony PCR
LacP-torA-GFP-DT (1-8) LacP-pspA-DT (1-8)
| 2×Quick Taq | VF2 | VR | MilliQ | Total |
|---|---|---|---|---|
| 25 | 1 | 1 | 23 | 50 |
94℃, 2min
94℃, 30sec
55℃, 30sec
68℃, 1.5min
→30cycles
Restriction
by Terasaka
| J23107-tatABCD | EcoR1 | Spe1 | BufferM | BSA | MilliQ | total |
|---|---|---|---|---|---|---|
| 10 | 0.5 | 0.5 | 3 | 0.5 | 15.5 | 30 |
| GFP-DT | Xba1 | Pst1 | BufferM | BSA | MilliQ | total |
|---|---|---|---|---|---|---|
| 15 | 0.5 | 0.5 | 3 | 0.5 | 10.5 | 30 |
Electrophoresis
① -⑧. LacP-pspA-DT
1-8. LacP-torA-GFP-DT
Gel extraction
- J23107-tatABCD(EcoR1, Spe1)
- J23107-tatABCD(Pst1, Spe1) ①57.6μg/mL ②23.4μg/mL
- GFP-DT(Xba1, Pst1)
Restriction
| pspA-DT(145.5μg/mL) | Xba1 | Pst1 | BufferM | BSA | MilliQ | total |
|---|---|---|---|---|---|---|
| 20 | 0.5 | 0.5 | 3 | 0.5 | 5.5 | 30 |
37℃, overnight
Liquid culture
- LacP-torA-GFP-DT-7 (LB 2mL)
+IPTG 0mM, 0.05mM, 0.1mM, 0.15mM, 0.2mM, 0.3mM
- LacP-torA-GFP-DT-7 (LB 5mL)
September 8
Check the effect of Amp
We used 5mL of the liquid culture medium with LacP-torA-GFP-DT-7.
→See "September 7 -Liquid culture-"
We diluted it with LB until OD600=0.444, and dispensed it.
The dispense volume was 1.3mL.
We added 0/13/130μL of Amp(50mg/mL) to it.
5.5h after incubating at 37℃
| Amp | 0μL | 13μL | 130μL |
|---|---|---|---|
| OD600 | 2.457 | 2.696 | 0.310 |
Observation through a confocal microscope
We used the liquid culture media with LacP-torA-GFP-DT + 13μL of Amp(50mg/mL) + IPTG 0/0.1/0.15mM.
Their OD600 was 0.47.
3h after incubating at 37℃, we eliminated each supernatant using a centrifuge, and diluted them with LB to which Amp was added.
2.5h after incubating at 37℃, we observed them through a confocal microscope.
We failed to observe it.
Gel extraction
pspA-DT -5.5μg/mL
Restriction
| pspA-DT-3(322μg/mL) | Xba1 | Pst1 | BufferM | BSA | MilliQ | total |
|---|---|---|---|---|---|---|
| 10 | 1 | 1 | 3 | 3 | 12 | 30 |
at 37℃ for 1h
September 9
Restriction
| J23107-tatABCD(102.9μg/mL) | EcoR1 | Spe1 | BufferM | MilliQ | total |
|---|---|---|---|---|---|
| 10 | 1 | 1 | 3 | 15 | 30 |
Electrophoresis
1. J23107-tatABCD(EcoR1, Spe1)
2. J23107-tatABCD(before restriction)
3. pspA-DT(Xba1, Pst1)
4. pspA-DT(before restriction)
Gel extraction
- J23107-tatABCD(EcoR1, Spe1) 26μg/mL
- pspA-DT(Xba1, Pst1) 199μg/mL
Liquid culture
- J23100/J23101/J23106-1
- pspA-DT-5 ×2
- LacP-torA-GFP-DT-21
- GFP generator-2
- LacP-pspA-DT-6
September 10
Miniprep
- J23100-1 105.5μg/mL
- J23101-1 76.0μg/mL
- J23106-1 90.9μg/mL
- pspA-DT-5 ①104.1μg/mL ②80.0μg/mL
- LacP-torA-GFP-DT-21 79.6μg/mL
- GFP generator-2 84.1μg/mL
- LacP-pspA-DT-6 99.8μg/mL
Ligation
- J23107-tatABCD-DT
| J23107-tatABCD(EcoR1, Spe1) | DT(EcoR1, Xba1) | Ligation High Ver.2 | total |
|---|---|---|---|
| 10 | 5 | 7.5 | 22.5 |
- Negative control for J23107-tatABCD-DT
| DT(EcoR1, Xba1) | Ligation High Ver.2 | total |
|---|---|---|
| 5 | 5 | 10 |
- J23107-tatABCD-pspA-DT
| J23107-tatABCD(Spe1, Pst1) | pspA-DT(Xba1, Pst1) | Ligation High Ver.2 | total |
|---|---|---|---|
| 3 | 6 | 9 | 18 |
- Negative control for J23107-tatABCD-pspA-DT
| J23107-tatABCD(Spe1, Pst1) | Ligation High Ver.2 | total |
|---|---|---|
| 3 | 3 | 6 |
Restriction
| DNA | Spe1 | Pst1 | BufferH | MilliQ | total |
|---|---|---|---|---|---|
| 15 | 1 | 1 | 3 | 10 | 30 |
DNA=J23100(105.5μg/mL), J23101(76.0μg/mL), J23106(90.9μg/mL)
at 37℃
Gel extraction
J23100 27.1μg/mL
J23101 50.2μg/mL
J23106 16.1μg/mL
Ligation
- J23100/J23101/J23106-kil-DT
| J23100/J23101/J23106(Spe1, Pst1) | kil-DT(Xba1, Pst1) | Ligation High Ver.2 | total |
|---|---|---|---|
| 1 | 5 | 3 | 9 |
- Negative control for J23100/J23101/J23106-kil-DT
| J23100/J23101/J23106(Spe1, Pst1) | MilliQ | Ligation High Ver.2 | total |
|---|---|---|---|
| 1 | 5 | 3 | 9 |
Transformation
J23100/J23101/J23106-kil-DT
September 11
Colony PCR
- J23107-tatABCD-DT (1-8)
- J23107-tatABCD-pspA-DT (1-8)
| 2×Quick Taq | VF2 | VR | MilliQ | Total |
|---|---|---|---|---|
| 25 | 1 | 1 | 23 | 50 |
94℃, 2min
94℃, 30sec
55℃, 30sec
68℃, 3.5min
→30cycles
Electrophoresis
- J23107-tatABCD-DT (1-8)
- J23107-tatABCD-pspA-DT (1-8)
Restriction
| GFP generator(84.1μg/mL) | Xba1 | Pst1 | BufferM | BSA | MilliQ | total |
|---|---|---|---|---|---|---|
| 15 | 1 | 1 | 3 | 3 | 7 | 30 |
| LacP-torA-GFP-DT(85.0μg/mL) | Spe1 | Pst1 | BufferH | MilliQ | total |
|---|---|---|---|---|---|
| 15 | 1 | 1 | 3 | 10 | 30 |
| LacP-pspA-DT(99.8μg/mL) | Xba1 | Pst1 | BufferM | BSA | MilliQ | total |
|---|---|---|---|---|---|---|
| 15 | 1 | 1 | 3 | 3 | 7 | 30 |
Liquid culture
pSB1C3(self-ligation) ×5 in LB 3mLCP 3μL
When OD600=approximately 0.6, we added 0/30/60/150/300μL of Amp(50mg/mL) to each liquid culture medium.
1h and 5h after incubating at 37℃, we measured OD600.
| 1h | 5h | |
|---|---|---|
| 0 | -0.020 | 0.403 |
| 30 | -0.046 | 0.544 |
| 60 | 0.263 | 0.007 |
| 150 | 0.061 | 0.839 |
| 300 | 0.093 | 1.441 |
Ligation
| pSB3C5(EcoR1, Pst1) | pspA-DT(Xba1, Pst1) | J23107-tatABCD(EcoR1, Spe1) | Ligation High Ver.2 | total |
|---|---|---|---|---|
| 1 | 3 | 5 | 4.5 | 13.5 |
4℃, overnight
Colony PCR
- J23100-kil-DT (1-6)
- J23101-kil-DT (1-7)
- J23106-kil-DT (1-2)
| 2×Quick Taq | VF | VR | MilliQ | Total |
|---|---|---|---|---|
| 23 | 1 | 1 | 25 | 50 |
Gel extraction
- GFP generator (Xba1, Pst1) 14.1μg/mL
- LacP-torA-GFP-DT (Spe1, Pst1) 42.9μg/mL 1.13(260/280) 1.19(260/230)
- LacP-pspA-DT (Xba1, Pst1) 20.0μg/mL 1.01(260/280) 1.30(260/230)
Electrophoresis
- J23107-tatABCD-DT (1-8)
- J23106-kil-DT (1, 2)
- J23100-kil-DT (1-4)
Liquid culture
- LacP-torA-GFP-DT
- pSB1C3
Colony PCR
We did PCR 10 more cycle to amplify products of PCR on 9/11 of J23107-tatABCD-pspA-DT and J23107-tatABCD-DT.
September 12
Colony PCR
J23107-tatABCD-pspA-DT (9-15)
94℃, 2min
94℃, 30sec
55℃, 30sec
68℃, 4min
→26cycles
Check the effect of Amp
We cultured pSB1C3 for overnight.
→See "September 7 -Liquid culture-"
We diluted it with LB, and dispensed it.
We added Amp(50mg/mL) to it until density of Amp was 1/10, 1/30, 1/50 .
| Amp | start | 5 hours |
|---|---|---|
| 1/10 | 0.557 | 0.077 |
| 1/30 | 0.628 | 0.166 |
| 1/50 | 0.472 | 0.055 |
| 0 | 0.594 | 2.585 |
Electrophoresis
- J23107-tatABCD-pspA-DT
- J23101-kil-DT (1-7)
Restriction
| pspA(pSB3C5) (106μg/mL) | EcoR1 | Pst1 | BufferH | MilliQ | total |
|---|---|---|---|---|---|
| 15 | 1 | 1 | 3 | 10 | 30 |
| pspA-DT-3 | Xba1 | Pst1 | BufferM | BSA | MilliQ | total |
|---|---|---|---|---|---|---|
| 15 | 1 | 1 | 3 | 3 | 7 | 30 |
| J23107-tatABCD (102.4μg/mL) | EcoR1 | Spe1 | BufferM | MilliQ | total |
|---|---|---|---|---|---|
| 15 | 1 | 1 | 3 | 10 | 30 |
Electrophoresis
- pspA(pSB3C5)(EcoR1, Pst1)
- pspA-DT(Xba1, Pst1)
- J23107-tatABCD(EcoR1, Spe1)
- pSB1C3(EcoR1, Spe1)
Transformation
J23107-tatABCD-pspA-DT(pSB3C5)
| DNA | Competent cell | total |
|---|---|---|
| 2 | 20 | 22 |
on ice for 30 minites heatshock at 42℃ for 1 hour on ice 2 minites then add SOC medium 200μl preculture at 37℃ for 1 hour incubate CP culture plate
Gel extraction
- pspA(pSB3C5)(EcoR1, Pst1) 26.8μg/mL 1.27(260/280) 0.32(260/230)
- pspA-DT(Xba1, Pst1) 26.1μg/mL 1.13(260/280) 0.55(260/230)
- J23107-tatABCD(EcoR1, Spe1) 1.5μg/mL 1.99(260/280) 0.06(260/230)
Ligation
| pSB3C5(EcoR1, Pst1) 26.8μg/mL | pspA-DT(Xba1, Pst1) 26.1μg/mL | J23107-tatABCD(EcoR1, Spe1) 1.5μg/mL | Ligation High Ver.2 | total |
|---|---|---|---|---|
| 1 | 3 | 6 | 5 | 15 |
| pSB3C5(EcoR1, Pst1) 26.8μg/mL | MilliQ | Ligation High Ver.2 | total |
|---|---|---|---|
| 1 | 9 | 5 | 15 |
| LacP(pSB3C5)(Spe1, Pst1) 10μg/mL | GFP-DT(Xba1, Pst1) 7.0μg/mL | Ligation High Ver.2 | total |
|---|---|---|---|
| 1 | 5 | 3 | 9 |
| LacP(pSB3C5)(Spe1, Pst1) 10μg/mL | MilliQ | Ligation High Ver.2 | total |
|---|---|---|---|
| 1 | 5 | 3 | 9 |
4℃, overnight
Observation through a confocal microscope
Evernoteに上がっている写真をお願いします。
September 13
Miniprep
- J23107-tatABCD-pspA-DT(pSB3C5)-1 130μg/mL 1.37(260/280) 0.79(260/230)
Restriction
| J23107-tatABCD-pspA-DT(pSB3C5) (130μg/mL) | Spe1 | Pst1 | BufferH | MilliQ | total
|
|---|---|---|---|---|---|
| 10 | 1 | 1 | 3 | 15 | 30 |
Transformation
- LacP-GFP generater
- LacP
- J23107-tatABCD-pspA-DT(pSB3C5)
- J23107-tatABCD
Colony PCR
J23107-tatABCD-pspA-DT
→See "September 14 -Colony PCR-"
September 14
Colony PCR
See "September 13 -Colony PCR-"
J23107-tatABCD-pspA-DT
Colony PCR
- J23107-tatABCD-pspA-DT (1-6)
| 2×Quick Taq | VF | VR | MilliQ | Total |
|---|---|---|---|---|
| 23 | 1 | 1 | 25 | 50 |
94℃, 2min
94℃, 30sec
55℃, 30sec
68℃, 4min
→30cycles
September 15
Liquid culture
- J23107-tatABCD-pspA-DT-1
- J23107-tatABCD-pspA-DT-2
- J23107-tatABCD-pspA-DT-3
Miniprep
- J23107-tatABCD-pspA-DT-1 22.9μg/mL
- J23107-tatABCD-pspA-DT-2 51.8μg/mL 1.57(260/280) 1.01(260/230)
- J23107-tatABCD-pspA-DT-3 28.5μg/mL 1.57(260/280) 0.94(260/230)
Restriction
| J23107-tatABCD-pspA-DT (51.8μg/mL) | EcoR1 | Spe1 | BufferM | MilliQ | total |
|---|---|---|---|---|---|
| 15 | 0.5 | 0.5 | 3 | 11 | 30 |
| LacP-torA-GFP-DT (79.6μg/mL) | EcoR1 | Xba1 | BufferM | BSA | MilliQ | total |
|---|---|---|---|---|---|---|
| 10 | 0.5 | 0.5 | 3 | 0.5 | 15.5 | 30 |
| LacP-kil-DT (56.0μg/mL) | EcoR1 | Xba1 | BufferM | BSA | MilliQ | total |
|---|---|---|---|---|---|---|
| 15 | 0.5 | 0.5 | 3 | 0.5 | 10.5 | 30 |
| J23107-tatABCD-pspA-DT (51.8μg/mL) | EcoR1 | Pst1 | BufferM | MilliQ | total |
|---|---|---|---|---|---|
| 15 | 0.5 | 0.5 | 3 | 11 | 30 |
37℃, 2 hours
Electrophoresis
①. 1kb ladder
②. J23107-tatABCD-pspA-DT
③. J23107-tatABCD-pspA-DT(EcoR1, Spe1)
④. J23107-tatABCD-pspA-DT(EcoR1, Pst1)
⑤. LacP-kil-DT
⑥. LacP-kil-DT(EcoR1, Xba1)
⑦. LacP-torA-GFP-DT
⑧. LacP-torA-GFP-DT(EcoR1, Xba1)
Gel extraction
①. 1kb ladder
②. J23107-tatABCD-pspA-DT(EcoR1, Spe1)
③. J23107-tatABCD-pspA-DT(EcoR1, Spe1)
④. J23107-tatABCD-pspA-DT(EcoR1, Pst1)
⑤. J23107-tatABCD-pspA-DT(EcoR1, Pst1)
⑥. 1kb ladder
- J23107-tatABCD-pspA-DT(EcoR1, Spe1) 14.2μg/mL 1.28(260/280) 0.54(260/230)
- J23107-tatABCD-pspA-DT(EcoR1, Pst1) 7.3μg/mL 1.55(260/280) 0.38(260/230)
Purification
- LacP-torA-GFP-DT(EcoR1, Xba1) 7.7μg/mL 1.41(260/280) 0.99(260/230)
- LacP-kil-DT(EcoR1, Xba1) →failed
Ligation
| LacP-torA-GFP-DT(EcoR1, Xba1) | J23107-tatABCD-pspA-DT(EcoR1, Spe1) | Ligation High Ver.2 | total |
|---|---|---|---|
| 4 | 10 | 7 | 21 |
| pSB1C3(EcoR1, Pst1) | J23107-tatABCD-pspA-DT(EcoR1, Pst1) | Ligation High Ver.2 | total |
|---|---|---|---|
| 4 | 10 | 7 | 21 |
at 16℃ for overnight
September 16
Transformation
LacP-torA-GFP-DT-J23107-tatABCD-pspA-DT
J23107-tatABCD-pspA-DT(pSB1C3)
September 17
Colony PCR
J23107-tatABCD-pspA-DT-LacP-torA-GFP-DT (1-6)
J23107-tatABCD-pspA-DT(pSB1C3) (1-6)
J23107-kil-DT (6-10)
Restriction
| LacP-torA-GFP-DT (126μg/mL) | EcoR1 | Xba1 | BufferM | BSA | MilliQ | total |
|---|---|---|---|---|---|---|
| 10 | 1 | 1 | 3 | 3 | 12 | 30 |
| LacP-torA-GFP-DT (126μg/mL) | Xba1 | BufferM | BSA | MilliQ | total |
|---|---|---|---|---|---|
| 3 | 1 | 3 | 3 | 20 | 30 |
Electrophoresis
A1-A6. J23107-tatABCD-pspA-DT-LacP-torA-GFP-DT (1-6)
B1-B6. J23107-tatABCD-pspA-DT(pSB1C3) (1-6)
Liquid culture
- J23107-tatABCD-pspA-DT(pSB1C3)
- J23107-tatABCD-pspA-DT(pSB3C5)
- LacP-torA-GFP-DT(pSB3C5)
Electrophoresis
写真お願いします。
lane1. 1kb ladder
lane2. J23101-kil-DT-6
lane3. J23101-kil-DT-7
lane4. J23101-kil-DT-8
lane5. J23101-kil-DT-10
lane6. LacP-torA-GFP-DT
lane7. LacP-torA-GFP-DT(EcoR1, Xba1)
lane8. LacP-torA-GFP-DT(Xba1)
Miniprep
- J23106-kil-DT 11.7μg/mL 1.93(260/280) 1.46(260/230)
September 18
Gel extraction
- LacP-torA-GFP-DT(EcoR1, Xba1) 36.9μg/mL 1.37(260/280) 0.82(260/230)
Ligation
| J23107-tatABCD-pspA-DT(EcoR1, Spe1) 14.2μg/mL | LacP-torA-GFP-DT(EcoR1, Xba1) 36.9μg/mL | Ligation High Ver.2 | total |
|---|---|---|---|
| 8 | 1.5 | 9.5 | 19 |
| LacP-torA-GFP-DT(EcoR1, Xba1) 36.9μg/mL | Ligation High Ver.2 | total |
|---|---|---|
| 1.5 | 1.5 | 3 |
at 4℃ for 1 hour
Transformation
- J23107-tatABCD-pspA-DT-LacP-torA-GFP-DT
- LacP-torA-GFP-DT(EcoR1, Xba1)
Observation through a confocal microscope
We made sample for observation through a confocal microscope.
We incubated sample with 0mM/0.5mM IPTG for 1.5 hours.
Then, we incubated onto medium without IPTG for 2.5 hours.
| IPTG(mM) | medium |
|---|---|
| 0 | LB |
| 0.1 | LB |
| 0.1 | LB with 0.1mM IPTG (positive control) |
| 0 | LB with 2 percent glucose |
| 0.1 | LB with 2 percent glucose |
| 0 | LB with 5 percent glucose |
| 0.1 | LB with 5 percent glucose |
| 0 | LB with 2 percent glucose (make medium new after incubate for 1.5 hours) |
| 0 | LB with 2 percent glucose (make medium new after incubate for 1.5 hours) |
Restriction
| pSB4K5 | EcoR1 | Pst1 | BufferH | MilliQ | total |
|---|---|---|---|---|---|
| 10 | 1 | 1 | 3 | 15 | 30 |
| pSB4K5 | EcoR1 | BufferH | MilliQ | total |
|---|---|---|---|---|
| 3 | 0.5 | 1 | 5.5 | 30 |
| pSB4K5 | Pst1 | BufferH | MilliQ | total |
|---|---|---|---|---|
| 3 | 0.5 | 1 | 5.5 | 10 |
| LacP-torA-GFP-DT | Xba1 | Pst1 | BufferM | BSA | MilliQ | total |
|---|---|---|---|---|---|---|
| 10 | 1 | 1 | 3 | 3 | 12 | 30 |
| LacP-torA-GFP-DT | Xba1 | BufferM | BSA | MilliQ | total |
|---|---|---|---|---|---|
| 2 | 1 | 1 | 1 | 5 | 10 |
| LacP-torA-GFP-DT | Pst1 | BufferH | MilliQ | total |
|---|---|---|---|---|
| 2 | 1 | 1 | 6 | 10 |
37℃, 2 hours
Gel extraction
- LacP-torA-GFP-DT(Xba1, Pst1) 9.7μg/mL 1.34(260/280) 0.62(260/230)
- pSB4K5(EcoR1, Pst1) 9.6μg/mL 1.28(260/280) 0.32(260/230)
Miniprep
- J23107-tatABCD-psp-DT(pSB1C3) 78μg/mL
- J23107-tatABCD-psp-DT(pSB1C3) 48μg/mL
- LacP-torA-GFP-DT 29μg/mL
- J23101-kil-DT 25μg/mL
Restriction enzyme processing
| J23101-Kil-DT 25µg/ml | BufferM | Xba1 | Pst1 | BSA | MilliQ | total |
|---|---|---|---|---|---|---|
| 40 | 6 | 1 | 1 | 6 | 6 | 60 |
| J23101-Kil-DT 25µg/ml | BufferM | Xba1 | BSA | MilliQ | total |
|---|---|---|---|---|---|
| 3 | 1 | 0.5 | 1 | 4.5 | 10 |
| J23101-Kil-DT 25µg/ml | BufferH | Pst1 | MilliQ | total |
|---|---|---|---|---|
| 3 | 1 | 0.5 | 5.5 | 60 |
at 37℃ for 2 hours
Electrophoresis
写真お願いします。
①. pSB4K5
②. pSB4K5(Pst1)
③. pSB4K5(EcoR1)
④. pSB4K5(EcoR1, Pst1)
⑤. LacP-torA-GFP-DT
⑥. LacP-torA-GFP-DT(Xba1)
⑦. LacP-torA-GFP-DT(Pst1)
⑧. LacP-torA-GFP-DT(Xba1, Pst1)
写真お願いします。
①. 1kb ladder
②. J23101-Kil-DT(Xba1, Pst1)
③. J23101-Kil-DT(Xba1, Pst1)
④. J23101-Kil-DT(Xba1, Pst1)
⑤. J23101-Kil-DT(Xba1, Pst1)
⑥. 1kb ladder
Restriction enzyme processing
| J23101-Kil-DT | BufferM | Xba1 | Pst1 | BSA | MilliQ | total |
|---|---|---|---|---|---|---|
| 15 | 3 | 1 | 1 | 3 | 7 | 30 |
| J23101-Kil-DT | BufferM | Xba1 | BSA | MilliQ | total |
|---|---|---|---|---|---|
| 3 | 1 | 0.5 | 1 | 4.5 | 10 |
| J23101-Kil-DT | BufferH | Pst1 | MilliQ | total |
|---|---|---|---|---|
| 3 | 1 | 0.5 | 5.5 | 10 |
37℃
September 19
GeneClean II
LacP-torA-GFP-DT
4k5
Ligation
| LacP-torA-GFP-DT(Xba1,Pst1) 9.7μg/mL | pSB4K5(EcoR1,Pst1) 9.6μg/mL | J23107-tatABCD-PsPA-DT(EcoR1,Spe1) 14.2μg/mL | Ligation High Ver.2 | total |
|---|---|---|---|---|
| 7 | 2 | 7 | 8 | 24μL |
| pSB4K5(EcoR1,Pst1) 9.6μg/mL | Ligation High Ver.2 | MilliQ | total |
|---|---|---|---|
| 2 | 1 | 21 | 24μL |
incubate at 37℃ for 2h
Electrophoresis
J23101-kil-DT(Xba1,Pst1) (Xba1) (Pst1)
Lane1: 1kb ladder
Lane2: empty
Lane3: plasmid
Lane4: Xba1
Lane5: Pst1
Lane6: Xba1,Pst1
Liquid Culture
We did liquid culture J23106-kil-DT(9/10, Amp)'s 6,7 at LB mediumAmp.
Transformation
J23107-tatABCD-pspA-DT-LacP-torA-GFP-DT
pSB4K5 negative control
Liquid culture
J23107-tatABCD-pspA-DT-LacP-torA-GFP-DT:1~5
Colony PCR
J23107-tatABCD-pspA-DT-LacP-torA-GFP-DT
| VF2 | VR | Quick tag | MilliQ | total |
|---|---|---|---|---|
| 1 | 1 | 25 | 23 | 50 |
Predenature 94℃ 2min
Denature 94℃ 30sec
Annealing 55℃ 30sec
Extension 68℃ 5min
→30cycles
Restriction enzyme processing
| LacP-kil-DT(4) 62.5μg/mL | Xba1 | Pst1 | 10xBSA | MilliQ | BufferM | total |
|---|---|---|---|---|---|---|
| 10 | 1 | 1 | 3 | 12 | 3 | 30 |
| LacP-kil-DT(4) | EcoR1 | Pst1 | MilliQ | BufferH | total |
|---|---|---|---|---|---|
| 10 | 1 | 1 | 15 | 3 | 30 |
| LacP-kil-DT | Xba1 | 10xBSA | MilliQ | BufferM | total |
|---|---|---|---|---|---|
| 2 | 0.5 | 1 | 5.5 | 1 | 10 |
| LacP-kil-DT | EcoR1 | MilliQ | BufferH | total |
|---|---|---|---|---|
| 2 | 0.5 | 6.5 | 1 | 10 |
| LacP-kil-DT | Pst1 | MilliQ | BufferH | total |
|---|---|---|---|---|
| 2 | 0.5 | 6.5 | 1 | 10 |
at 37℃, 2 hours
Electrophoresis
Lane1: 1kb ladder
Lane2: empty
Lane3: plasmid
Lane4: EcoR1
Lane5: Xba1
Lane6: Pst1
Lane7: EcoR1,Pst1
Lane8: Xba1,Pst1
Electrophoresis
J23107-tatABCD-pspA-DT-LacP-torA-GFP-DT
Lane1: ladder
Lane2: 1
Lane3: 2
Lane4: 3
Lane5: 4
Lane6: 5
Ligation
| LacP-torA-GFP-DT(Xba1,Pst1) 9.7μg/mL | pSB4K5(EcoR1,Pst1) 9.6μg/mL | J23107-tatABCD-PsPA-DT(EcoR1,Spe1) 14.2μg/mL | Ligation High Ver.2 | total |
|---|---|---|---|---|
| 7 | 4 | 7 | 9 | 27μL |
(Negative control)
| pSB4K5(EcoR1,Pst1) 9.6μg/mL | Ligation High Ver.2 | MilliQ | total |
|---|---|---|---|
| 4 | 9 | 14 | 27μL |
Restriction enzyme processing
| torA-R9 101.8μg/mL | bufferH | MilliQ | EcoR1 | Pst1 | total |
|---|---|---|---|---|---|
| 10 | 3 | 15 | 1 | 1 | 30 |
| torA-R9 101.8μg/mL | bufferH | MilliQ | EcoR1 | Pst1 | total |
|---|---|---|---|---|---|
| 3 | 1 | 5.5 | 0.5 | 0 | 10 |
| torA-R9 101.8μg/mL | bufferH | MilliQ | EcoR1 | Pst1 | total |
|---|---|---|---|---|---|
| 3 | 1 | 5.5 | 0 | 0.5 | 10 |
PCR
| torA-R9 | MilliQ | 10xPCR buffer for KOD plus neo | 2mM dNTP3 | 25mM MgSO4 | M13 | KOD plus neo | total |
|---|---|---|---|---|---|---|---|
| 0.5 | 34 | 5 | 5 | 3 | 1.5 | 1 | 50 |
Predenature 94℃ 2min
Denature 98℃ 10sec
Annealing 60℃ 30sec
Extension 68℃ 20sec
→35cycles
Electrophoresis
torA-R9,plasmid,(EcoR1),(Pst1),(EcoR1,Pst1)
Lane1: 1kb ladder
Lane2: empty
Lane3: plasmid
Lane4: EcoR1
Lane5: Pst1
Lane6: EcoR1,Pst1
Lane7: empty
Lane8: 1kb ladder
Liquid culture
LacP-tatA-GFP-DT (9/4)
PCR purification
torA-R9 68.0μg/mL
September 20
Gel extraction
LacP-kil-DT (EcoR1,Pst1) (Xba1,Pst1)
Restriction enzyme processing
| torA-R9 68.0μg/mL | bufferM | Spe1 | MilliQ | Total |
|---|---|---|---|---|
| 20 | 3 | 1 | 6 | 30 |
at 37℃, 2h
Ligation
| LacP-kil-DT(Xba1,Pst1) 12.4μg/mL | pSB1C3(EcoR1,Pst1) 34.2μg/mL | J23107-tatABCD-PsPA-DT(EcoR1,Spe1) 14.2μg/mL | Ligation High Ver.2 | total |
|---|---|---|---|---|
| 7 | 4 | 7 | 9 | 27μL |
(Negative control)
| pSB1C3(EcoR1,Pst1) | Ligation High Ver.2 | MilliQ | total |
|---|---|---|---|
| 4 | 9 | 14 | 27μL |
Transformation
Using above Ligation and Negative control's product and torA-R9.
Miniprep
J23106-kil-DT 6; 12.0μg/mL, (260/280): 1.78, (260/230): 1.73
J23106-kil-DT 7; 13.6μg/mL, (260/280): 1.85, (260/230): 1.73
Restriction enzyme processing
| GFP-DT 84.1μg/mL | bufferM | 10xBSA | BbaI | MilliQ | total |
|---|---|---|---|---|---|
| 15 | 3 | 3 | 1 | 8 | 30 |
at 37℃, 2 hours
Colony PCR
We made J23107-tatABCD-pspA-DT-LacP-torA-GFP-DT's 1~8 samples.
| Quick tag | tat f primer | VR | MilliQ | total |
|---|---|---|---|---|
| 25 | 1 | 1 | 23 | 50 |
Predenature 94℃ 2min
Denature 94℃ 30sec
Annealing 54℃ 30sec
Extension 68℃ 4min
→30cycles
Liquid culture
We did liquid culture J23107-tatABCD-pspA-DT-LacP-torA-GFP-DT's 1~4 samples.
Electrophoresis
J23701-tatABCD-pspA-DT-LacP-torA-GFP-DT
Lane1: 1kb ladder
Lane2: 1
Lane3: 2
Lane4: 3
Lane5: 4
Lane6: 5
Lane7: 6
Lane8: 7
Lane9: 8
Lane10: 1kb ladder
Lane11: empty
Lane12: empty
torA-R9 (EcoR1,Pst1): 31μg/mL, (280/280): 0.80, (280/230): 0.39
pSB1C3(EcoR1,Pst1): 22μg/mL, (280/280):1.33, (280/230):0.6
torA-R9 (Spe1): 56μg/mL, (280/280): 1.11, (280/230): 0.69
GFP-DT(Xba1): 45μg/mL, (280/280): 1.15, (280/230): 0.88
Ligation
| torA-R9 (EcoR1,Pst1) | pSB1C3(EcoR1,Pst1) | Ligation High Ver.2 | total |
|---|---|---|---|
| 8 | 2 | 5 | 15μL |
| torA-R9 (Spe1) | pSB1C3(Xba1) | Ligation High Ver.2 | total |
|---|---|---|---|
| 3 | 3 | 3 | 9μL |
at 16℃, 1hour
Colony PCR
J23107-tatABCD-pspA-DT-LacP-torA-GFP-DT (4k5)
numbering 9~15
Transformation
J23107-tatABCD-pspA-DT-LacP-torA-GFP-DT (4k5)
J23107-tatABCD-pspA-DT-LacP-torA-kil-DT(pSB1C3)
Negative control (pSB1C3)
Negative control(pSB4K5)
torA-R9(pSB1C3)
Liquid culture
J23107-tatABCD-pspA-DT-LacP-torA-GFP-DT: colony PCR(9~15)
| 2xQuick tag | VF | VR | MilliQ | total |
|---|---|---|---|---|
| 25 | 1 | 1 | 23 | 50 |
Predenature 94℃ 2min
Denature 94℃ 30sec
Annealing 55℃ 30sec
Extension 68℃ 5min
→30cycles
PCR
| torA-R9-GFP-DT | MilliQ | KOD plus ver.2 buffer | dNTP3 | MgSO4 | M13 | VR | KOD plus | total |
|---|---|---|---|---|---|---|---|---|
| 1 | 35 | 5 | 5 | 2 | 1.5 | 1.5 | 1 | 52 |
Predenature 94℃ 2min
Denature 94℃ 15sec
Annealing 55℃ 30sec
Extension 68℃ 5min
→30cycles
Miniprep
J23701-tatABCD-pspA-DT-LacP-torA-GFP-DT:2,3
2: 10.4μg/mL, (250/280): 1.45, (260/230): 0.95
3: 6.0μg/mL, (250/280): 1.32, (260/230): 0.79
(*)100μL
Electrophoresis
torA-R9-GFP-DT(after PCR)
September 21
Gel extraction
torA-R9-GFP-DT(after PCR): 10.3μg/mL, (260/280): 1.28, (220/230): 0.49
Electrophoresis
J23107-tatABCD-pspA-DT-LacP-torA-GFP-DT(kan): 9~15
Electrophoresis
J23107-tatABCD-pspA-DT-LacP-torA-GFP-DT: 1,2
Lane1: empty
Lane2: empty
Lane3: 2
Lane4: 3
Lane5: 1kb ladder
Restriction enzyme processing
| LacP-kil-DT(3) | Xba1 | Pst1 | 10xBSA | MilliQ | BufferM | total |
|---|---|---|---|---|---|---|
| 15 | 1 | 1 | 3 | 7 | 3 | 30 |
| LacP-torA-GFP-DP(9/5) | Xba1 | Pst1 | 10xBSA | MilliQ | BufferM | total |
|---|---|---|---|---|---|---|
| 15 | 1 | 1 | 3 | 7 | 3 | 30 |
| LacP-kil-DT | Xba1 | 10xBSA | MilliQ | BufferM | total |
|---|---|---|---|---|---|
| 2 | 0.5 | 1 | 5.5 | 1 | 10 |
| LacP-torA-GFP-DP | Xba1 | 10xBSA | MilliQ | BufferM | total |
|---|---|---|---|---|---|
| 2 | 0.5 | 1 | 5.5 | 1 | 10 |
| LacP-kil-DT | Pst1 | MilliQ | BufferH | total |
|---|---|---|---|---|
| 2 | 0.5 | 6.5 | 1 | 10 |
| LacP-torA-GFP-DP | Xba1 | Pst1 | MilliQ | BufferH | total |
|---|---|---|---|---|---|
| 2 | 0.5 | 1 | 6.5 | 1 | 10 |
| J23107-tatABCD-pspA-DT | Spe1 | Pst1 | BufferH | MilliQ | total |
|---|---|---|---|---|---|
| 20 | 1 | 1 | 4 | 14 | 40 |
| J23107-tatABCD-pspA-DT | Spe1 | BufferM | MilliQ | total |
|---|---|---|---|---|
| 2 | 0.5 | 1 | 6.5 | 10 |
| J23107-tatABCD-pspA-DT | Pst1 | BufferH | MilliQ | total |
|---|---|---|---|---|
| 2 | 0.5 | 1 | 6.5 | 10 |
at 37℃, 2hours
September 22
Miniprep
torA-R9(Amp)1: 113.7μg/mL, (260/280): 1.96, (280/230): 2.42
torA-R9(Amp)2: 97.8μg/mL, (260/280): 1.67, (280/230): 1.72
torA-R9(Amp)3: 104.2μg/mL, (260/280): 1.93, (280/230): 2.67
LacP-torA-GFP-DT 6: 37.4μg/mL, (260/280): 2.24, (280/230): 3.27
Electrophoresis
LaneA: J23107-tatABCD (9/6)
LaneB: J23107-tatABCD-pspA-DT pSB3C5 (9/13)
LaneC: J23107-tatABCD-pspA-DT 1C5 (9/15)
LaneD: J23107-tatABCD-pspA-DT (9/15)
LaneE: J23107-tatABCD-pspA-DT(pSB1C3) (9/18)
LaneF: J23107-tatABCD-pspA-DT(pSB3C5) (9/18)
LaneG: J23107-tatABCD-pspA-DT-LacP-torA-GFP-DT:2 (9/20)
LaneH: J23107-tatABCD-pspA-DT-LacP-torA-GFP-DT:3 (9/20)
LaneA~H are (EcoR1,Pst1)
Restriction enzyme processing
| J23107-tatABCD-pspA-DT-LacPtorA-GFP-DT:2 | BufferH | Pst1 | MilliQ | total |
|---|---|---|---|---|
| 15 | 3 | 1 | 12 | 30 |
| J23107-tatABCD-pspA-DT-LacP-torA-GFP-DT:3 | BufferH | Pst1 | MilliQ | total |
|---|---|---|---|---|
| 15 | 3 | 1 | 12 | 30 |
Restriction enzyme processing
| LacP-torA-GFP-DT(37.4μg/mL) | BufferH | EcoR1 | Pst1 | total |
|---|---|---|---|---|
| 25 | 3 | 1 | 1 | 30 |
Electrophoresis
1: J23107-tatABCD-pspA-DT-LacP-torA-GFP-DT:2
2: LacP-torA-GFP-DT (EcoR1,Pst1) (9/22)
3: J23107-tatABCD-pspA-DT-LacP-torA-GFP-DT:3
September 23
Electrophoresis
写真お願いします。
- LacP-torA-GFP-DT(EcoR1, Pst1)
lane_1 1kb ladder lane_3 DNA lane_4 DNA lane_5 DNA
Gel extraction
写真お願いします。
- J23107-tatABCD-pspA-DT(pSB1C3)(Spe1, Pst1)
- LacP-torA-GFP-DT(Xba1, Pst1)
lane_1 1kb ladder
lane_3 J23107-tatABCD-pspA-DT(pSB1C3)(Spe1, Pst1)
lane_5 LacP-torA-GFP-DT(Xba1, Pst1)
Restriction
| LacP-torA-GFP-DT (78.4μg/mL) | Xba1 | Pst1 | BufferM | BSA | MilliQ | total |
|---|---|---|---|---|---|---|
| 15 | 1 | 1 | 3 | 3 | 7 | 30 |
| LacP-torA-GFP-DT (78.4μg/mL) | Xba1 | BufferM | BSA | MilliQ | total |
|---|---|---|---|---|---|
| 3 | 0.5 | 1 | 1 | 4.5 | 10 |
| J23107-tatABCD-pspA-DT(pSB1C3) (78μg/mL) | Spe1 | Pst1 | BufferH | MilliQ | total |
|---|---|---|---|---|---|
| 9 | 1 | 1 | 2 | 7 | 20 |
Ligation
| LacP-torA-GFP-DT(EcoR1, Pst1) (5.5μg/mL) | pSB4K5 (9.6μg/mL) | Ligation High Ver.2 | total |
|---|---|---|---|
| 8 | 4 | 6 | 18 |
at 16℃ for 1 hour
Liquid culture
- LacP-tatABCD-pspA-DT (2, 3, 5, 6)
Colony PCR
LacP-tatABCD-pspA-DT (1-6)
| 2×Quick Taq | VF2 | VR | MilliQ | Total |
|---|---|---|---|---|
| 25 | 1 | 1 | 23 | 50 |
94℃, 2min
94℃, 30sec
55℃, 30sec
68℃, 4min
→30cycles
Electrophoresis
写真お願いします。
①, LacP-torA-GFP-DT
②, LacP-torA-GFP-DT(Xba1, Pst1)
③, LacP-torA-GFP-DT(Xba1)
④, LacP-torA-GFP-DT(Pst1)
⑤, J23107-tatABCD-pspA-DT(Spe1, Pst1)
Liquid culture
- LacP-torA-GFP-DT (4, 7)
- LacP-GFP generater (1-3)
- T7-6His-GFP-DT (1, 2)
Gel extraction
写真お願いします。
- LacP-torA-GFP-DT (Xba1, Pst1) 6.8μg/mL 1.27(260/280) 0.40(260/230)
Purification
- J23107-tatABCD-pspA-DT(Spe1, Pst1) 15.1μg/mL 1.49(260/280) 0.80(260/230)
Ligation
| J23107-tatABCD-pspA-DT(Spe1, Pst1) (15.1μg/mL) | LacP-torA-GFP-DT (Xba1, Pst1) (6.8μg/mL) | Ligation High Ver.2 | total |
|---|---|---|---|
| 10 | 4 | 7 | 21 |
| J23107-tatABCD-pspA-DT(Spe1, Pst1) (15.1μg/mL) | LacP-kil-DT (Xba1, Pst1) (12.4μg/mL) | Ligation High Ver.2 | total |
|---|---|---|---|
| 8 | 2 | 5 | 15 |
at 16℃ for 1 hour
Transformation
LacP-torA-GFP-DT(pSB4K5)
J23107-tatABCD-pspA-DT-LacP-torA-GFP-DT
J23107-tatABCD-pspA-DT-LacP-kil-DT
| DNA | competent cell | total |
|---|---|---|
| 2 | 10 | 12 |
preculture at 37℃ for 1 hour culture at 37℃ for 16 hours
Electrophoresis
写真お願いします。
J23107-tatABCD-pspA-DT(pSB1C3) (1-6)
lane_1 1kb ladder
lane_2~7 DNA
lane_8 1kb ladder
September 24
Miniprep
- J23107-tatABCD-pspA-DT-2 139.4μg/ml 1.69(260/280) 1.83(260/230)
- J23107-tatABCD-pspA-DT-3 134.7μg/ml 1.61(260/280) 1.69(260/230)
- J23107-tatABCD-pspA-DT-5 139.3μg/ml 1.55(260/280) 1.45(260/230)
- J23107-tatABCD-pspA-DT-6 138.5μg/ml 1.58(260/280) 1.40(260/230)
- LacP-torA-GFP-DT-4
- LacP-torA-GFP-DT-7
- T7-6His-GFP-DT-1
- T7-6His-GFP-DT-2
Colony PCR
LacP-GFP generater (1-3)
| 2×Quick Taq | VF2 | VR | MilliQ | Total |
|---|---|---|---|---|
| 25 | 1 | 1 | 23 | 50 |
94℃, 2min
94℃, 30sec
55℃, 30sec
68℃, 1min
→30cycles
写真お願いします。
①-③, LacP-GFP generater (1-3)
Liquid culture
- J23107-tatABCD-pspA-DT-LacP-torA-GFP-DT (1-4)
- J23107-tatABCD-pspA-DT-LacP-kil-DT (1-3)
Colony PCR
J23107-tatABCD-pspA-DT-LacP-torA-GFP-DT (1-4)
J23107-tatABCD-pspA-DT-LacP-kil-DT (1-3)
| 2×Quick Taq | VF2 | VR | MilliQ | Total |
|---|---|---|---|---|
| 25 | 1 | 1 | 23 | 50 |
94℃, 2min
94℃, 30sec
55℃, 30sec
68℃, 5min
→30cycles
Electrophoresis
写真お願いします。
lane1, 1kb ladder
lane2-5, J23107-tatABCD-pspA-DT-LacP-torA-GFP-DT (1-4)
lane6-8, J23107-tatABCD-pspA-DT-LacP-kil-DT (1-3)
September 25
Liquid culture
- J23107-tatABCD-pspA-DT-LacP-torA-GFP-DT(pSB4K5)-2
Miniprep
J23107-tatABCD-pspA-DT-LacP-kil-DT(pSB1C3)-3: 26.2μg/mL, (260/280): 1.50, (280/230): 0.65
J23107-tatABCD-pspA-DT-LacP-torA-GFP(pSB1C3)-3: 24.8μg/mL, (260/280): 1.56, (280/230): 0.76
LacP-GFP generater-1: 42.0μg/mL, (260/280): 1.58, (280/230): 0.94
Restriction enzyme processing
| J23107-tatABCD-pspA-DT-LacP-torA-GFP-DT(pSB1C3) (26.2ng/µL) | EcoRl | Spel | buffer M | MiliQ | Total |
|---|---|---|---|---|---|
| 15 | 1 | 1 | 3 | 10 | 30 |
| J23107-tatABCD-pspA-DT-LacP-torA-GFP-DT(pSB1C3) (26.2ng/µL) | EcoRl | buffer M | MiliQ | Total |
|---|---|---|---|---|
| 2 | 0.5 | 1 | 6.5 | 10 |
| J23107-tatABCD-pspA-DT-LacP-torA-GFP-DT(pSB1C3) (26.2ng/µL) | Spel | buffer M | MiliQ | Total |
|---|---|---|---|---|
| 2 | 0.5 | 1 | 6.5 | 10 |
| J23107-tatABCD-pspA-DT-LacP-kil-DT(pSB1C3) (24.8ng/µL) | EcoRl | Pstl | buffer H | MiliQ | Total |
|---|---|---|---|---|---|
| 15 | 1 | 1 | 3 | 10 | 30 |
| J23107-tatABCD-pspA-DT-LacP-kil-DT(pSB1C3) (24.8ng/µL) | Pstl | buffer H | MiliQ | Total |
|---|---|---|---|---|
| 2 | 0.5 | 1 | 6.5 | 10 |
| J23107-tatABCD-pspA-DT-LacP-kil-DT(pSB1C3) (24.8ng/µL) | EcoRl | buffer H | MiliQ | Total |
|---|---|---|---|---|
| 2 | 0.5 | 1 | 6.5 | 10 |
at 37℃ for 2 hours
Electrophoresis
写真お願いします。
lane1, 1kb ladder
lane2, J23107-tatABCD-pspA-DT-LacP-torA-GFP-DT(pSB1C3)
lane3, J23107-tatABCD-pspA-DT-LacP-torA-GFP-DT(pSB1C3) (EcoRl, Spel)
lane4, J23107-tatABCD-pspA-DT-LacP-torA-GFP-DT(pSB1C3) (EcoRl)
lane5, J23107-tatABCD-pspA-DT-LacP-torA-GFP-DT(pSB1C3) (Spel)
lane6, J23107-tatABCD-pspA-DT-LacP-kil-DT(pSB1C3)
lane7, J23107-tatABCD-pspA-DT-LacP-kil-DT(pSB1C3) (EcoRl, Pstl)
lane8, J23107-tatABCD-pspA-DT-LacP-kil-DT(pSB1C3) (EcoRl)
lane9, J23107-tatABCD-pspA-DT-LacP-kil-DT(pSB1C3) (Pstl)
lane10, LacP-torA-GFP-DT(EcoR1,Xba1)(9/15)
lane11, LacP-torA-GFP-DT(EcoR1,Xba1)(9/17)
lane12, 1kb ladder
→The bands didn't appear, so we did one more electrophoresis.
写真お願いします。
lane1, 1kb ladder
lane2, LacP-torA-GFP-DT(EcoR1,Xba1)(9/17)
lane3, J23107-tatABCD-pspA-DT-LacP-torA-GFP-DT(pSB1C3)
lane4, J23107-tatABCD-pspA-DT-LacP-torA-GFP-DT(pSB1C3) (EcoRl, Spel)
lane5, J23107-tatABCD-pspA-DT-LacP-torA-GFP-DT(pSB1C3) (EcoRl)
lane6, J23107-tatABCD-pspA-DT-LacP-torA-GFP-DT(pSB1C3) (Spel)
Liquid culture
- LacP-torA-GFP-DT(pSB4K5) (1-4)
Restriction enzyme processing
| J23107-tatABCD-pspA-DT-LacP-torA-GFP-DT(pSB1C3) | EcoRl | Spel | buffer M | MiliQ | Total |
|---|---|---|---|---|---|
| 15 | 1 | 1 | 3 | 10 | 30 |
| J23107-tatABCD-pspA-DT-LacP-kil-DT(pSB1C3) | EcoRl | Pstl | buffer H | MiliQ | Total |
|---|---|---|---|---|---|
| 15 | 1 | 1 | 3 | 10 | 30 |
at 37℃ for 1 hour
Electrophoresis
J23107-tatABCD-pspA-DT-LacP-torA-GFP-DT(pSB1C3) (EcoRl, Spel)
J23107-tatABCD-pspA-DT-LacP-kil-DT(pSB1C3) (EcoRl, Pstl)
→The bands didn't appear.
 "
"