Team:Peking/Project/Phototaxis/Design
From 2012.igem.org
| (19 intermediate revisions not shown) | |||
| Line 10: | Line 10: | ||
<div class="PKU_context floatR first"> | <div class="PKU_context floatR first"> | ||
| - | <p>CheZ is dephosphorylase of CheY in chemotaxis pathway. Three main characteristics actuate our consideration of CheZ. First, several experiments indicate that CheZ can effect the movement of bacteria | + | <h3 id="title1">Design</h3> |
| + | <p>CheZ is the dephosphorylase of CheY in chemotaxis pathway. Three main characteristics actuate our consideration of CheZ for our usage. First, several experiments indicate that CheZ can effect the movement of bacteria, and increasing levels of CheZ induced by arabinose can enlarge the diameter of swarming colonies. CheZ deletion causes cells to tumble incessantly, resulting in a nonmotile phenotype in semisolid agar, and reintroducing CheZ restores cell motility. Second, CheZ is commonly used as motility-control module in synthetic biology. A combination of CheZ and the quorum sensing LuxI/R part forms stripe pattern in colony. Bacteria with CheZ under the control of an atrazine binding riboswitch proves the capability of atrazine chemotaxis. CheZ controlled by theophylline riboswitch of upstream random sequence acts as a colony reporter for orthogenesis. Furthermore, CheZ is included in some downsteam locomotive part of bacteria phototaxis such as in <i>Halobacterium</i>. On this basis, we decided to use CheZ to control the mobility of the bacteria.<br/><br/> | ||
| + | |||
| + | Through rational consideration, we embark on building the circuit. CheZ can only function at a high expression level, so we linked CheZ with the strong promoter <a href="http://partsregistry.org/Part:BBa_B0034">RBS B0034</a> and placed it in a high copy plasmid. We design a light-on system to regulate the CheZ expression: Without light, CI repress the CheZ expression;When illuminated by blue light, CI expression is repressed by luminesensor so that the CheZ expression increases to enhance the bacterial motility.</p> | ||
<div class="floatC"> | <div class="floatC"> | ||
| - | <img src="/wiki/images/ | + | <img src="/wiki/images/8/88/Hposhvposdijpdoigj.png" alt="Figure 1." style="width:600px"/> |
| - | <p class="description">Figure 1. | + | <p class="description" style="text-align:center;width:350px;">Figure 1. Gene circuit for Phototaxis.</p> |
</div> | </div> | ||
| - | <p>After gathering principles and parameters of chemotaxis system, we then simulated the phototaxis system in a stochastic way to reflectively describe the phototaxis mechanism of our system | + | <p>After gathering principles and parameters of chemotaxis system, we then simulated the phototaxis system in a stochastic way to reflectively describe the phototaxis mechanism of our system(See <a href="https://2012.igem.org/Team:Peking/Modeling/Phototaxis">Modeling Phototaxis</a>).</p> |
<div class="floatC"> | <div class="floatC"> | ||
| - | + | ||
| - | + | ||
</div> | </div> | ||
| - | <p>As the modeling indicated, we designed several experiments to realize phototaxis. Firstly, we have to verify the function of CheZ. Two experiments were designed: the measurement of diameter of mobile (MG1655) and immobile (DH5 α and ΔCheZ MG1655) bacteria and the bacteria with CheZ under different strength of promoters: BBa_J23112, BBa_J23113, and BBa_J23114, whose relative expression levels of downstream gene are expected to be 1, 21, and 256, respectively. Sceondly, plates pasted polaroid of different transmittance are used to measure CheZ expression under different light strength. Thirdly, plot the bacteria in the center of the plate whose half is opaque. These experiments together are expected to have different diameter of colony or oval shape of colony to show the phototaxis. | + | <p>As the modeling indicated, we designed several experiments to realize phototaxis. Firstly, we have to verify the function of CheZ. Two experiments were designed: the measurement of diameter of mobile (MG1655) and immobile (DH5 α and ΔCheZ MG1655) bacteria and the bacteria with CheZ under different strength of promoters: <a href="http://partsregistry.org/Part:BBa_J23112">BBa_J23112</a>, <a href="http://partsregistry.org/Part:BBa_J23113">BBa_J23113</a>, and <a href="http://partsregistry.org/Part:BBa_J23114">BBa_J23114</a>, whose relative expression levels of downstream gene are expected to be 1, 21, and 256, respectively. Sceondly, plates pasted polaroid of different transmittance are used to measure CheZ expression under different light strength. Thirdly, plot the bacteria in the center of the plate whose half is opaque. These experiments together are expected to have different diameter of colony or oval shape of colony to show the phototaxis. |
</p> | </p> | ||
| + | </div> | ||
| + | <div class="PKU_context floatR"> | ||
| + | <h3 id="title1">Reference</h3> | ||
| + | <p></p> | ||
| + | <ul class="refer"><li id="ref1"> | ||
| + | 1. Kuo, S.C., and Koshland, D.E.(1987) Roles of che Y and cheZ Gene Products in Controlling Flagellar Rotation in Bacterial Chemotaxis of <i>Escherichia coli</i>. <i>J. Bacteriol.</i>, 3:1307:1313 | ||
| + | </li><li id = "ref2"> | ||
| + | 2. Bren, A., Welch, M., Blat, Y., Eisenbeach, M.(1996) Signal termination in bacterial chemotaxis: CheZ mediates dephosphorylation of free rather than switch-bound CheY. <i>Proc. Natl. Acad. Sci. USA</i>, 93: 10090: 10093 | ||
| + | </li><li id = "ref3"> | ||
| + | 3. Bren, A., and Eisenbeach, M.(2000) How Signals Are Heard during Bacterial Chemotaxis: Protein-Protein Interactions in Sensory Signal Propagation. <i>J. Bacteriol.</i>, 182: 6865: 6873 | ||
| + | </li><li id = "ref4"> | ||
| + | 4. Sanna, M. G., and Simon, M.I.(1996) <i>in vivo</i> and <i>in vitro</i> Characterization of <i>Escherichia coli</i> Protein CheZ Gain- and Loss-of-Function Mutants. <i>J. Bacteriol.</i>, 178: 6275: 6280 | ||
| + | </li><li id = "ref5"> | ||
| + | 5. Liu, C., <i>et al</i>.(2012) Sequential Establishment of Stripe Patterns in an Expanding Cell Population. <i>Science</i>, 334: 238: 241 | ||
| + | </li><li id = "ref6"> | ||
| + | 6. Lee, S.H., Butler, S.M., and Camilli, A.(2001) Selection for in vivo regulators of bacterial virulence. <i>Proc. Natl. Acad. Sci. USA</i> 98: 6889: 6894 | ||
| + | </li><li id = "ref7"> | ||
| + | 7. sinha, J., Reyes, S.J., Gallivan, J.P.(2010) Reprogramming bacteria to seek and destroy an herbicide. <i>Nat. Chem. Biol.</i>, 464:468 | ||
| + | </li><li id = "ref8"> | ||
| + | 8. Topp, S., and Gallivan. J.P.(2008) Random Walks to Synthetic Riboswitches — A High-Throughput Selection Based on Cell Motility. <i>Chem. Biol.</i>, 9:210:213 | ||
| + | </li></ul> | ||
</div> | </div> | ||
</html> | </html> | ||
{{Template:Peking2012_Color_Epilogue}} | {{Template:Peking2012_Color_Epilogue}} | ||
Latest revision as of 03:02, 27 October 2012
Design
CheZ is the dephosphorylase of CheY in chemotaxis pathway. Three main characteristics actuate our consideration of CheZ for our usage. First, several experiments indicate that CheZ can effect the movement of bacteria, and increasing levels of CheZ induced by arabinose can enlarge the diameter of swarming colonies. CheZ deletion causes cells to tumble incessantly, resulting in a nonmotile phenotype in semisolid agar, and reintroducing CheZ restores cell motility. Second, CheZ is commonly used as motility-control module in synthetic biology. A combination of CheZ and the quorum sensing LuxI/R part forms stripe pattern in colony. Bacteria with CheZ under the control of an atrazine binding riboswitch proves the capability of atrazine chemotaxis. CheZ controlled by theophylline riboswitch of upstream random sequence acts as a colony reporter for orthogenesis. Furthermore, CheZ is included in some downsteam locomotive part of bacteria phototaxis such as in Halobacterium. On this basis, we decided to use CheZ to control the mobility of the bacteria.
Through rational consideration, we embark on building the circuit. CheZ can only function at a high expression level, so we linked CheZ with the strong promoter RBS B0034 and placed it in a high copy plasmid. We design a light-on system to regulate the CheZ expression: Without light, CI repress the CheZ expression;When illuminated by blue light, CI expression is repressed by luminesensor so that the CheZ expression increases to enhance the bacterial motility.
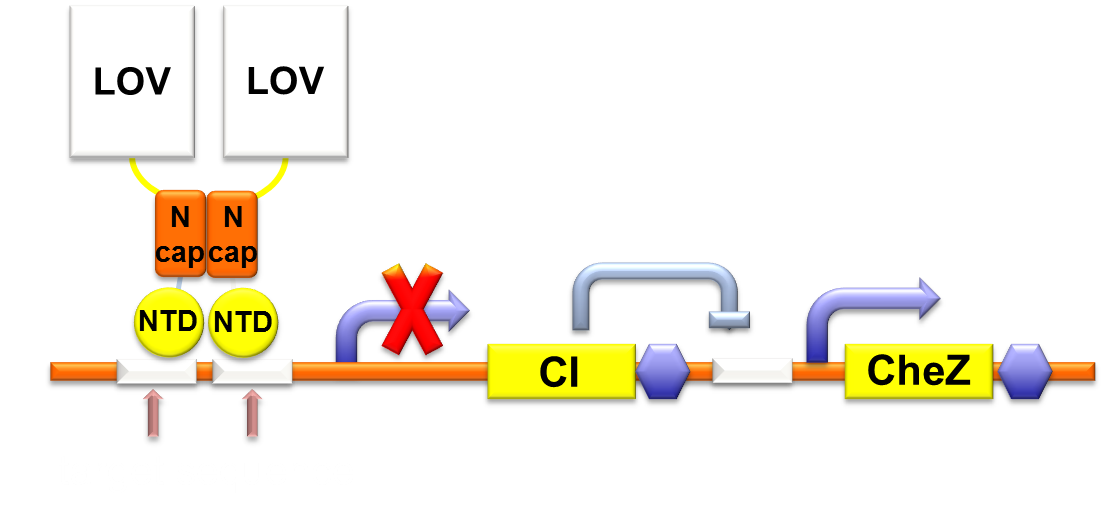
Figure 1. Gene circuit for Phototaxis.
After gathering principles and parameters of chemotaxis system, we then simulated the phototaxis system in a stochastic way to reflectively describe the phototaxis mechanism of our system(See Modeling Phototaxis).
As the modeling indicated, we designed several experiments to realize phototaxis. Firstly, we have to verify the function of CheZ. Two experiments were designed: the measurement of diameter of mobile (MG1655) and immobile (DH5 α and ΔCheZ MG1655) bacteria and the bacteria with CheZ under different strength of promoters: BBa_J23112, BBa_J23113, and BBa_J23114, whose relative expression levels of downstream gene are expected to be 1, 21, and 256, respectively. Sceondly, plates pasted polaroid of different transmittance are used to measure CheZ expression under different light strength. Thirdly, plot the bacteria in the center of the plate whose half is opaque. These experiments together are expected to have different diameter of colony or oval shape of colony to show the phototaxis.
Reference
- 1. Kuo, S.C., and Koshland, D.E.(1987) Roles of che Y and cheZ Gene Products in Controlling Flagellar Rotation in Bacterial Chemotaxis of Escherichia coli. J. Bacteriol., 3:1307:1313
- 2. Bren, A., Welch, M., Blat, Y., Eisenbeach, M.(1996) Signal termination in bacterial chemotaxis: CheZ mediates dephosphorylation of free rather than switch-bound CheY. Proc. Natl. Acad. Sci. USA, 93: 10090: 10093
- 3. Bren, A., and Eisenbeach, M.(2000) How Signals Are Heard during Bacterial Chemotaxis: Protein-Protein Interactions in Sensory Signal Propagation. J. Bacteriol., 182: 6865: 6873
- 4. Sanna, M. G., and Simon, M.I.(1996) in vivo and in vitro Characterization of Escherichia coli Protein CheZ Gain- and Loss-of-Function Mutants. J. Bacteriol., 178: 6275: 6280
- 5. Liu, C., et al.(2012) Sequential Establishment of Stripe Patterns in an Expanding Cell Population. Science, 334: 238: 241
- 6. Lee, S.H., Butler, S.M., and Camilli, A.(2001) Selection for in vivo regulators of bacterial virulence. Proc. Natl. Acad. Sci. USA 98: 6889: 6894
- 7. sinha, J., Reyes, S.J., Gallivan, J.P.(2010) Reprogramming bacteria to seek and destroy an herbicide. Nat. Chem. Biol., 464:468
- 8. Topp, S., and Gallivan. J.P.(2008) Random Walks to Synthetic Riboswitches — A High-Throughput Selection Based on Cell Motility. Chem. Biol., 9:210:213
 "
"